Abstract
BACKGROUND AND PURPOSE: Recent advances in data-processing techniques have allowed more accurate MR-based volumetric measurement than was possible in the past. The purpose of this study was to use this technique to evaluate the development of the temporal lobes in childhood.
METHODS: The study group consisted of 42 subjects aged 3 weeks to 14 years (mean age, 5 years), all with normal findings on a routine MR study and none with a history of epilepsy. MR images were acquired on a 1.0-T system using a T1-weighted 3D ultrafast gradient-echo sequence. The volumes of the hippocampal formations and temporal lobes were measured by using a workstation, and the percentage of hippocampal formations in the temporal lobes was calculated. Myelination in the limbic system and related structures was also evaluated.
RESULTS: The volume of the hippocampal formations increased sharply until the age of 2 years, and continued to increase slowly thereafter. However, the percentage of hippocampal formations in the temporal lobes showed a negative correlation with age. The hippocampal formations on the right side were larger than those on the left in 38 cases (91%), and the anterior temporal lobes on the right were larger than those on the left in 32 cases (76%). This right-left asymmetry of the hippocampal formations and anterior temporal lobes was observed from early infancy, and these differences were statistically significant. A longitudinal fasciculus of high signal intensity was seen in the white matter beneath the subiculum by about 3 months of age.
CONCLUSION: MR-based volumetry established developmental characteristics of the temporal lobe, such as a hippocampal growth spurt, a growth difference between the hippocampal formation and the rest of the temporal lobe, and right-left asymmetry. Knowledge of these characteristics may aid in the understanding of hippocampal and temporal lobe abnormalities in children.
The temporal lobe and its components play a functional and/or etiologic role in language production, memory, and complex partial seizure disorders (1–3). The hippocampal formation, which is a component of the medial temporal lobe, has received particular attention owing to its role in seizure disorder and memory (4–8).
MR imaging has been used in biometric studies of the hippocampal formation and anterior temporal lobe for several years (9–19). To investigate the relationship between MR-based volumetric measurements of the temporal lobe and pathologic states involving the temporal lobes, Jack et al (9) established normative volumetric values of the right and left hippocampal formations and anterior temporal lobes in young adults aged 20 to 40 years. However, to our knowledge, no attempt has been made to describe the appearance and growth of the temporal lobes in infants and children.
Recently, abnormal hippocampal formations have been described in congenital brain anomalies in children, such as agenesis of the corpus callosum, lissencephaly, and holoprosencephaly, and these abnormalities have been thought to show incomplete development of the limbic system, including the hippocampal formations (20–22). Because knowledge of the normal developmental changes of the temporal lobe may help us understand the pathologic origins of the maldevelopment of the limbic system or other temporal lobe abnormalities, we decided to study the normal volumetric development of the temporal lobe, including the hippocampal formation, in childhood. In addition, we assessed myelination of the white matter fasciculus in the limbic system and its related structures.
Methods
Subjects
The study group consisted of 42 Japanese children, including 19 boys and 23 girls, ranging in age from 3 weeks to 14 years (mean age, 5 years). Clinical indications for MR imaging were suspected perinatal hypoxia (n = 12), migraine (n = 9), short stature (n = 6), cyanotic or syncope attack (n = 3), suspected seizure disorders (n = 3), macrocephaly (n = 3), dizziness attack (n = 2), suspected speech delay (n = 2), trauma (n = 1), and unilateral meatal atresia (n = 1). All subjects had normal neurologic development, and in all cases the routine MR findings were normal. Information about handedness was not available.
MR Acquisition
MR examinations were performed using a 1.0-T system with a quadrature head coil. Volumetric studies were performed using a T1-weighted 3D ultrafast gradient-echo sequence with preparation inversion pulse and the following parameters: 13–17/6–8/2 (TR/TE/excitations), flip angle, 25°; inversion time, 600; field of view, 180 to 230 mm; matrix, 256 × 205; slab thickness, 60 to 75 mm, and 40 to 50 partitions with an in-plane resolution of 0.9 × 1.1 mm. All data acquisition was performed in the coronal plane, which was perpendicular to the anterior commissure--posterior commissure (AC-PC) line, since this line is used as a stereotactic coordinate of neuroanatomy (23).
Volumetric Measurement
Each acquisition was transferred to a Sparc station 5 (Sun Microsystems, Mountain View, CA) and analyzed using Easy Vision CT/MR software (Release 2; Philips Medical Systems, Eindhoven, the Netherlands). From each set of volumetric data, coronal multiplanar reconstruction (MPR) images, which were perpendicular to the AC-PC line, were created with section intervals of 2 mm and a magnification factor of ×4. On these MPR images, one experienced neuroradiologist determined the area of interest by combining a thresholding technique, segmentation with a region-growing algorithm (24, 25) and manual tracing with a 3D mouse-guided cursor, which appeared simultaneously at the same location in all the active visualized planes; this device permits the operator to know the exact anatomic location of the cursor (19). The volumes of the right and left hippocampal formations and anterior temporal lobes were then calculated by counting voxels.
Boundaries of the Hippocampal Formation andTemporal Lobe
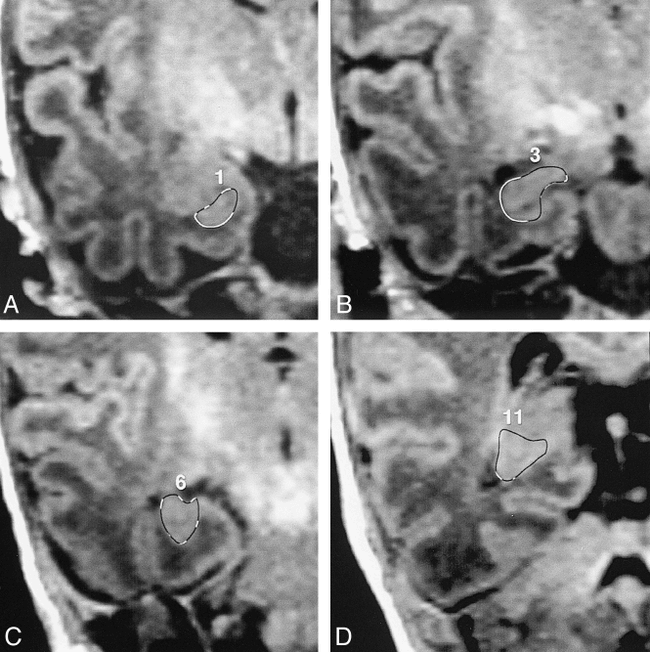
The boundaries of the hippocampal formation were outlined manually (19) (Fig 1). The ventral boundary was delineated by the white matter of the parahippocampal gyrus. Dorsally and laterally, the alveus or floor of the inferior horn provided a landmark for the hippocampal head. It almost always allows the observer to differentiate the hippocampal head from the overlying amygdala using a 3D cursor. However, in some of the neonates or young infants, in whom myelination of the alveus was incomplete, it was difficult to distinguish between these structures, and the observer had to make some arbitrary judgments regarding the boundaries between the most rostral part of the hippocampal head and amygdala. The amygdalohippocampal area was cut by a horizontal line to the alveus. At the level of the hippocampal body, we included the alveus overlying the cornu ammonis, fimbria, and dentate gyrus in the measurement. The dorsal and lateral boundary was also outlined by the alveus or floor of the inferior horn. Medially, we chose an arbitrary landmark located in the middle of the subiculum. To delineate a more caudal portion of the hippocampal formation, the origin of the crus fornices was cut along the extent of the alveus. The medial landmark was defined by a middle portion between the most lateral part of the hippocampus and the most medial part of the isthmus. The section that showed the entire length of the crus fornices was considered the posterior limit of the hippocampal tail.
Outlines of the hippocampal formation on coronal reconstructed images (perpendicular to the AC-PC line) in a 1-month-old girl.
A, Rostral part of the hippocampal head: the low intensity of the unmyelinated alveus demarcates the boundary between the hippocampal head and overlying amygdala.
B, Hippocampal head at the level of the posterior uncus: the dorsal boundary is outlined by the floor of the inferior horn.
C, Hippocampal body: The medial landmark is the arbitrary line in the middle of the subiculum.
D, Hippocampal tail: the medial boundary is cut through the middle between the most lateral part of the hippocampus and the most medial part of the isthmus.
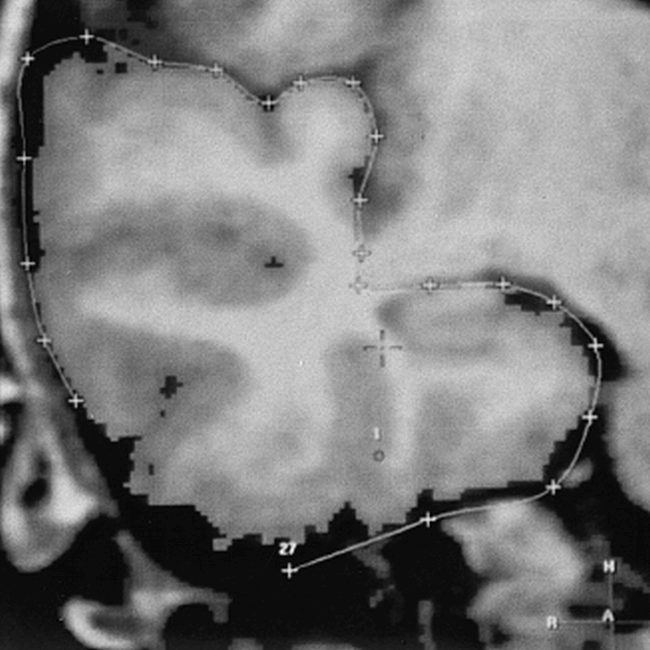
The right and left posterior boundaries of the temporal lobes were cut by coronal planes, which were perpendicular to the AC-PC line, that passed through the posterior limits of the hippocampal tails. On the anterior aspects, we outlined this structure using a thresholding technique, since the circumference of the temporal lobe is completely delineated by the subarachnoid space. More posteriorly, according to the protocol described by Jack et al (9), the temporal stem was manually separated from the overlying hemisphere by tracing a line that extended horizontally from medial to lateral through the choroidal fissure, vertically through the temporal stem from the temporal horn to the inferior aspect of the vertical potion of the sylvian fissure, and horizontally between the frontal and temporal opercula (Fig 2).
Outlines of the anterior temporal lobe using seed-point and region-growing algorithms on a coronal reconstructed image in an 8-year-old girl. The temporal stem was separated from the overlying hemisphere by tracing a line that extended horizontally from medial to lateral through the choroidal fissure, vertically through the temporal stem from the temporal horn to the inferior aspect of the vertical portion of the sylvian fissure, and horizontally between the frontal and temporal opercula
On the basis of the numerical values of volume obtained by the 3D software, we calculated the relative percentages of the right and left hippocampal formations within the whole anterior temporal lobes for each subject.
Myelination in the Limbic System and Related Structures
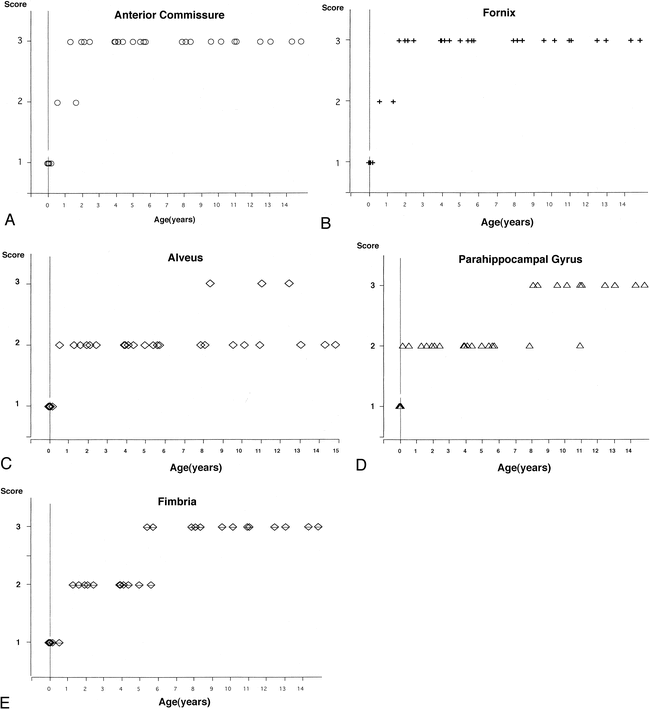
On the basis of the intensity of white matter relative to that of gray matter (intensity of the neocortex in the temporal lobe), we categorized myelination into three grades and scored each grade as follows: 1 = lower intensity than gray matter or isointensity (intensity equal to that of gray matter), 2 = higher intensity than gray matter, 3 = much higher intensity than gray matter (equal to the intensity of the posterior limb of the internal capsule or optic tract); higher scores indicated more complete myelination. Using this scoring system, we evaluated the major white matter in the limbic system and related structures, including the alveus, the fimbria, the fornix (body of fornix), and the white matter of the parahippocampal gyrus on coronal MPR images; the alveus, fimbria, and white matter of the parahippocampal gyrus were evaluated on sections that showed the hippocampal body. The anterior commissure was evaluated on a midsagittal MPR image. The mammillothalamic tract was not analyzed, since it could not be clearly delineated in some subjects.
Statistical Analysis
Pearson's correlation coefficient was used to evaluate the relationship between age and the relative percentages of the right and left hippocampal formations within the whole anterior temporal lobes. Wilcoxon's signed rank test was used to evaluate the right-left differences in volume of the hippocampal formations and anterior temporal lobes.
Results
Volumetric Measurements
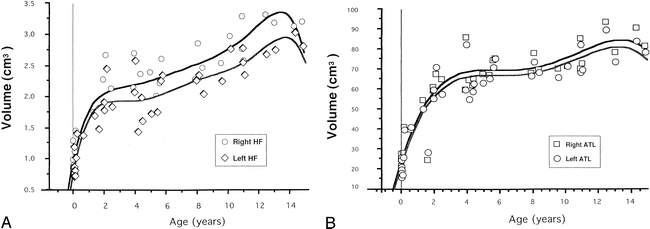
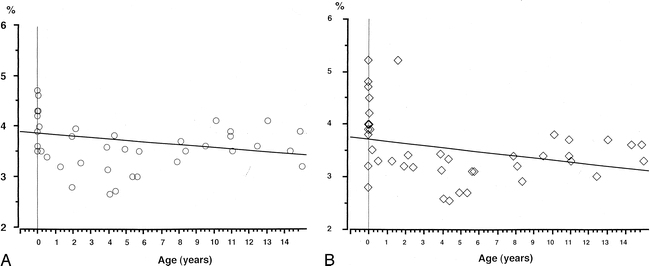
MR images showed that the hippocampal formation was infolded deeper into the temporal lobe and that the hippocampal sulcus was nearly obliterated in all of the subjects. Thus, from early infancy, the basic morphology of the hippocampal formation is the same as that in the adult (22). However, the volumes of the hippocampal formations and temporal lobes increased sharply until the age of 2 years, continuing to increase slowly thereafter (Fig 3). The percentages of both the right and left hippocampal formations in each temporal lobe were inversely related to age; this relationship was statistically significant on the left (r = −.308; P < .05) but not on the right (r = −.232; P = .13) (Fig 4).
A and B, Plots of volumes of hippocampal formations (A) and anterior temporal lobes (B) by age. The volumes increased sharply until the age of 2 years, and continued to increase slowly thereafter. HF indicates hippocampal formation; ATL signifies anterior temporal lobe
A and B, Correlation between the percentage of hippocampal formation in the temporal lobe and age. The percentage of both the right (A) and left (B) hippocampal formations in each temporal lobe showed a negative correlation with age
The hippocampal formations on the right side were larger than those on the left in 38 subjects (91%). In addition, the anterior temporal lobes on the right were larger than those on the left in 32 cases (76%). In all age groups, the mean volumes of the hippocampal formation and anterior temporal lobe on the right were larger than those on the left, and these differences were statistically significant (P < .05) in all age groups, except for the volumes of the anterior temporal lobes from 1 to 2 years of age (Table).
Volumetric measurements of the hippocampal formations and anterior temporal lobes in all age groups
Myelination
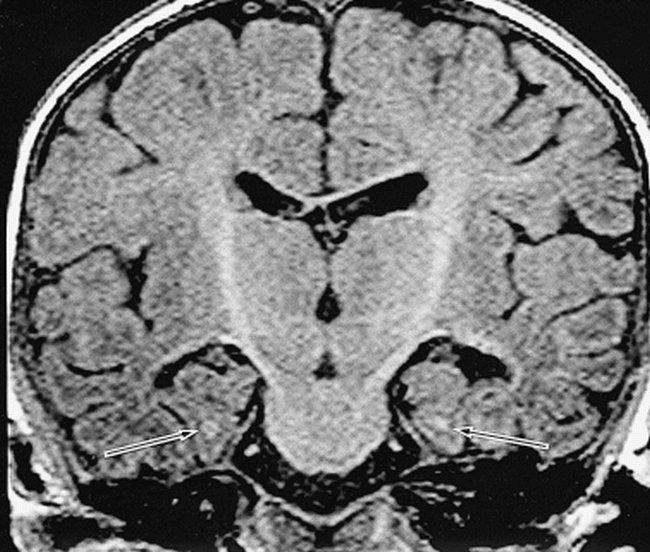
The myelination of white matter progressed until the age of 2 years. The anterior commissure and fornix reached the maximum score (ie, 3) before 2 years of age, while the other three fasciculi reached the maximum score after 5 years of age (Fig 5). We also observed the myelinated longitudinal fasciculus in the parahippocampal gyrus, which runs just beneath the subiculum, by about 3 months of age (Fig 6).
A–E, Myelination of the white matter in the limbic system and related structures. The anterior commissure (A) and fornix (B) reached the maximum score before 2 years of age, while the other three fasciculi–the alveus (C), parahippocampal gyrus (D), and fimbria (E)—reached the maximum score after 5 years of age
Longitudinal fasciculus in the parahippocampal gyrus in a 3-month-old boy. Coronal image shows the myelinated fasciculus beneath the subiculum (arrows)
Discussion
Development
The hippocampal formation refers to the functional unit consisting of the hippocampus (cornu ammonis), dentate gyrus, associated white matter (including the alveus, fimbria, and fornix), and subiculum (which comprises the medial and superior margins of the parahippocampal gyrus to the level of the hippocampus) (26, 27). Embryologically, the hippocampus belongs to the archicortex, which is part of the phylogenetically primitive cerebral allocortex and undergoes organization and maturation slower than the cerebral neocortex (21, 28–31). This differential growth of the neocortex relative to the allocortex results in rotation of the telencephalic cavity, and most of the hippocampus is carried ventrally to lie in the inferomedial aspect of the temporal lobe (21).
Kretschmann et al (32) reported that the volume of the human hippocampal formation increases dramatically in the second half of pregnancy, while a still larger increase occurs in the first to second postnatal year; the largest increase in the hippocampal volume occurs in the postnatal period. They also emphasized that this growth spurt reaches its maximum in the second postnatal month of normal development. Our results showed that the volume of the hippocampal formations increased sharply until the age of 2 years and continued to increase slowly thereafter (Fig 3). Therefore, the hippocampal formations undergo a growth spurt in early infancy. The volumetric development of the anterior temporal lobes was similar to that of the hippocampal formations. However, the percentages of the hippocampal formations in the temporal lobes showed a negative correlation with age; a significant correlation was seen in the left temporal lobe but not in the right (Fig 4). This finding also indicates that differential growth with developmental gradients between the archicortex of the hippocampus and temporal neocortex continues after the postnatal period, especially in the left temporal lobe.
Dobbing and Sands (33–35) identified a specific phase of rapid brain growth that coincides with vulnerability to nutritional and other growth restrictions. They also suggested that a normal brain growth spurt under good conditions may be a prerequisite for subsequent satisfactory bodily growth. Regarding their hypothesis, our finding that hippocampal and temporal growth spurts occur in early infancy may indicate that this period is not only important for neurological development related to functions of the limbic system but is also vulnerable to damage that may cause temporal lobe seizures.
Asymmetry
Recently, Jack et al (9) evaluated the right-left asymmetry of the temporal lobes in an adult population by MR volumetry and reported that the right anterior temporal lobe and hippocampal formation were significantly larger than those on the left in right- but not left-handed subjects. In the past, gross anatomic asymmetry has been reported in the temporal lobe. Since Geschwind and Levitsky (36) confirmed the presence of a larger left temporal planum, which is the area in the supratemporal plane behind Heschl's gyrus, in an autopsy study of 100 adult brains, such asymmetry has been associated with the presence of speech function lateralized to the left hemisphere. In 1975, Wada et al (37) found that the left temporal planum was larger than that on the right in most infants as well as adults. Although the relationship between the temporal planum and temporal lobe volume is unclear, we also found that the volumes of the hippocampal formations and anterior temporal lobes are asymmetric even in neonates and infants (prelanguage population) (see Table). This finding may support the notion that the presence of temporal lobe asymmetry exists before the development of speech and language function.
Since information about handedness was not established in this study, we did not address the relationship between asymmetry of the temporal lobe and lateralization of the speech function. However, MR-based volumetry in the immature temporal lobe should allow a more advanced investigation of the anatomic and functional relationships in the temporal lobe.
Myelination
Myelination is a continuously changing and accessible marker of maturation in the developing infant brain. Several authors (38–42) have documented the changes in MR corresponding to myelination of the white matter in the neonate and infant; a relatively normal adult appearance can be seen at age 2 years, and all major fiber tracts can be identified by age 3 years. Although our evaluation was limited to T1-weighted images, our MR findings were consistent with previous investigations. Myelination of the fornix and anterior commissure developed rapidly compared with other fasciculi, such as the alveus, fimbria, and white matter in the parahippocampal gyrus (Fig 5). This finding may reflect the fact that the fibers in the fornix and anterior commissure are more compact than those in other white matter tracts (43, 44). Meanwhile, the longitudinal fasciculus in the parahippocampal gyrus was clearly myelinated by about 3 months of age (Fig 6). This fasciculus runs just beneath the subiculum and seems to reach the entorhinal area of the temporal lobe; it therefore may be related to the circuit of Papez, in which fibers that extend from the anterior thalamic nucleus in turn project to the cingulum to reach the temporal lobe entorhinal area (45, 46). In the parahippocampal gyrus, there are many limbic fiber tracts other than the circuit of Papez, such as the polysynaptic intrahippocampal pathway, which involves the perforant path and Schaffer collaterals, the direct intrahippocampal pathway, and efferent fibers from the hippocampus, which can reach many cortical areas via the entorhinal and perirhinal cortices. In addition, the hippocampus receives afferent fibers from many areas that it projects to; one of these fibers is part of the circuit of Papez. Thus, it may be important to assess the myelination of the hippocampal formation and parahippocampal gyrus, since these limbic connections are believed to participate in the regulation of emotional behavior and to be integral to learning and memory processes (26, 46).
Conclusion
Using MR-based volumetry, we established developmental characteristics of the temporal lobe, such as a hippocampal growth spurt during early infancy, a growth difference between the hippocampal formation and the rest of the temporal lobe, and right-left asymmetry of the hippocampal formations and temporal lobes. We also found a fasciculus in the parahippocampal gyrus that myelinated early, which may be part of the circuit of Papez. Awareness of these normal developmental events in the temporal lobe is important for understanding neurologic development related to the limbic system and its impairment.
Footnotes
↵1 Address reprint requests to Hidetsuna Utsunomiya, MD, Department of Radiology, Fukuoka University School of Medicine, 7-45-1, Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan.
References
- Received July 1, 1998.
- Copyright © American Society of Neuroradiology