Abstract
Summary: Lipoblastomatosis is a locally infiltrative tumor of embryonic fat. We describe the MR appearance of cervical lipoblastomatosis with epidural extension. The initial MR study showed features of a soft-tissue mass; a subsequent MR examination, performed after chemotherapy, depicted the lesion as a typical lipoma of high signal intensity on T1-weighted images and of intermediate signal on T2-weighted sequences.
Lipoblastoma is a benign tumor of embryonic fat that occurs in infancy and childhood (1). It constitutes 3% of tumors arising from fat cells in infants. The male:female ratio is 3:1 (2). Lipoblastomatosis is more uncommon and represents a diffuse locally infiltrative form of lipoblastoma (3). The tumor may have a rapid rate of growth (4). Rarely, an embryonic lipoma may spontaneously transform into a mature lipoma (5). We present the MR imaging appearance of cervical lipoblastomatosis causing cord compression subsequent to epidural extension. We also report its change into a mature lipoma with typical MR fat signal characteristics after chemotherapy.
Case Report
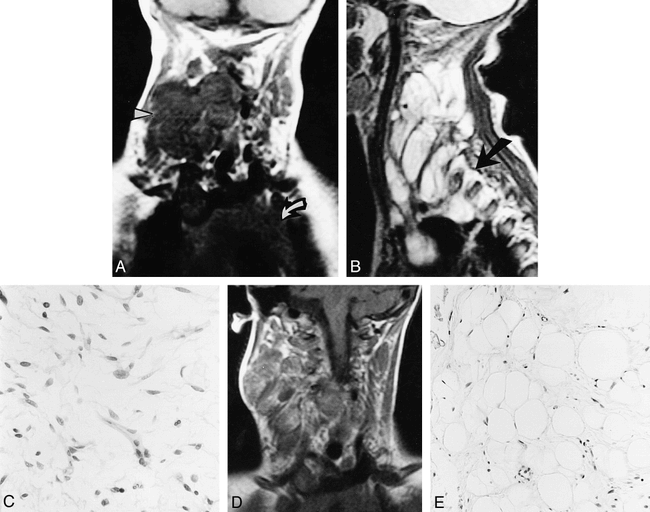
A 10-month-old girl was referred for assessment when her mother noticed that she was not using her right shoulder and arm. She had undergone a full-term normal delivery and had normal developmental milestones. Physical examination revealed torticollis, weakness, and wasting of the muscles of the right arm; otherwise, the infant was neurologically intact. MR imaging revealed a multilobular soft-tissue mass in the anterolateral aspect of the neck, deep to the sternomastoid muscle (Fig 1A and B). There was intraspinal tumor extension through dilated right intervertebral foramina from C2 to C5 vertebrae, causing moderate cord compression but no evidence of bone marrow infiltration. The mass was isointense with muscle on T1-weighted images (540/13/2 [TR/TE/excitations]) (Fig 1A); on T2-weighted fast spin-echo sequences (4000/90/2), the tumor had high-signal-intensity lobules, separated by thin bands of low signal (Fig 1B). Intense nonhomogeneous enhancement was noted after intravenous injection of gadopentetate dimeglumine.
10-month girl with torticollis, weakness, and wasting of the muscles of the right arm.
A, Coronal T1-weighted (540/13/2) MR image shows multilobular low-signal tumor on the right side of the neck (arrowhead) and superior mediastinum. Arrow indicates physiological thymic enlargement.
B, Sagittal T2-weighted (4000/90/2) image shows high-signal-intensity multilobular tumor with low-signal septa. The tumor spreads through the intervertebral foramina (arrow).
C, Representative section from the initial resection specimen shows cells with elongated, wispy cytoplasmic processes and variably shaped nuclei. Note absence of mitotic figures (original magnification ×250).
D, Coronal T1-weighted (540/13/2) follow-up MR examination shows increased signal intensity and bands of low signal within the tumor.
E, Representative section from the subsequent resection specimen shows the tumor now clearly consists of mature fat cells with relatively few nuclei (original magnification ×100).
The patient underwent exploration and debulking of the extradural component of a lobulated, soft, poorly circumscribed tumor. Microscopic examination of the tumor fragments revealed a lightly cellular neoplasm composed of cells with plump spindle-shaped nuclei and varying amounts of elongated and tapering eosinophilic cytoplasm. No mitotic figures were seen, but occasional cells with a bubbly appearance were noted. The tumor stroma consisted of thin wispy processes separated by empty spaces. A fibrocollagenous connective tissue capsule was present at the tumor edge. The appearance was interpreted as that of lipoblastomatosis (Fig 1C).
The patient improved postoperatively, but symptoms recurred and within 9 months torticollis and right hemiparesis developed again. A repeat MR examination revealed a significant increase in tumor size. The tumor was extensively debulked and the patient's weakness resolved. The histologic appearance was identical to that seen in the first excised specimen. In view of the aggressive nature of her symptoms, the patient was given a course of chemotherapy. She received six cycles of vincristine, ifosfamide, and actinomycin, according to the MMT 89 protocol.
Two years after chemotherapy, progressive swelling of the right side of the neck was noted. A repeat MR study confirmed the increase in size of the residual tumor. In addition, on the T1-weighted images, the tumor now showed a striking change, with a generalized increase in signal intensity interlaced with linear bands of low signal (Fig 1D). The patient again underwent debulking of the extraspinal tumor. Histologic examination at this stage revealed a multinodular lesion composed mostly of fat cells. The fatty lobules were separated from one another by thin strands of dense fibrocollagenous connective tissue. The recurrent tumor exhibited a significant decrease in cellularity and in the density of capillary vessels, and the fat cells appeared more mature in appearance as compared with the first biopsy specimen (Fig 1E). The overall appearance was that of a mature lipoma. Eighteen month later, the patient was well and neurologically intact, with normal developmental milestones.
Discussion
Lipoblastomas are tumors of mature embryonic fat and thus occur most often in infancy. Fifty-five percent of lipoblastomas arise before the age of 1 year. The tumor is rare after the age of 3, and has never been reported in children over the age of 8 years (3). Lipoblastomas are commonly well encapsulated but may be more diffuse and infiltrate through muscle planes, a condition known as lipoblastomatosis (2). The embryonic fat-cell precursors of the tumor, the lipoblasts, can still normally be found after birth in several locations, including the axilla, mediastinum, retroperitoneum, and prevertebral space. These areas are the most common sites for lipoblastomatosis (3).
While lipoblastomatosis infiltrates locally, it does not have a frank aggressive nature. Thus, it infiltrates along muscle planes without deep muscle invasion. These tumors have an excellent prognosis despite their occasional rapid growth to a considerable size (2). Calcification has never been reported (3), and metastases do not occur (2).
Soft-tissue tumors show signal characteristics in keeping with increased water content and thus appear dark on T1-weighted MR images and bright on T2-weighted images (6, 7), with a variable degree of contrast enhancement. This conforms with the initial appearance of the tumor in our case. This appearance reflects the histologic predominance of immature lipoblasts on initial imaging examinations; similar appearances have been described in lipoblastomatosis of the thigh (8).
Most soft-tissue tumors are difficult to differentiate on the basis of MR signal characteristics alone (6). The differential diagnosis commonly depends on their site of origin and on the patient's age at presentation. Neck masses arising in childhood are usually benign. Of those masses in which biopsies are performed, congenital lesions are found most frequently (55%), followed by inflammatory lymphadenopathy (30%), malignancy (10%), and benign neoplasm (5%) (9).
Congenital lesions include lymphangioma and branchial cysts. Lymphangiomas are most often of the cystic hygroma type. They occur on one side of the midline and may attain a considerable size (10). Branchial cysts are found in the anterior aspect of the infrahyoid part of the neck, deep to the sternomastoid muscle (11). On imaging, both lesions appear well defined and cystic (12). Soft-tissue enhancement may be seen in the hemangiomatous type of a lymphangioma (12) or with an infection (11). On MR images, tuberculous lymphadenitis may resemble a homogeneous soft-tissue mass, it may appear necrotic, or it may form matted masses of necrotic lymph nodes (11).
Benign neck tumors include schwannomas and neurofibromas. Schwannomas appear well encapsulated and may have a heterogeneous signal pattern on MR images owing to cystic or fatty degeneration. Neurofibromas may be multiple in von Recklinghausen disease and may form plexiform neurofibromas (11). These are widely infiltrative and tend to follow nerve roots. Infantile aggressive fibromatosis is characterized by uncontrolled proliferation of fibrous tissue and a tendency to recur locally after incomplete resection. Bone erosion is often present, and cord compression may develop. Despite apparent histologic differences, aggressive fibromatosis and congenital infantile fibrosarcoma behave similarly (9).
Lymphomas are the most common type of pediatric head and neck cancer (50%) followed by rhabdomyosarcoma (20%), nasopharyngeal carcinoma, and neuroblastoma (6%). Neuroblastoma that occurs in the neck is more likely to be due to lymph node secondaries of noncervical neuroblastoma. Ewing sarcoma and primitive neuroectodermal tumors may arise in the neck, from bone or soft tissues, and usually present in the first decade (9). The likely possibilities considered in our case included plexiform neurofibroma and lymphoma. A hemangiomatous lymphangioma may have been included, but intraspinal extension normally does not occur.
Alteration of signal intensity, usually with reduction of signal on T2-weighted images, occurs in soft-tissue tumors responding to chemotherapy (6) and in association with fibrosis after repeated surgery. The increased signal on T1-weighted images is unique to fatty change, hemorrhage (6), and sometimes calcification. In our patient, the manifestation of high signal on T1-weighted sequences on the subsequent MR examination was in keeping with the histologic findings of mature fat cells in the final biopsy specimen. The MR demonstration of mature cervical epidural lipoblastomatosis has been reported previously (1).
In general, a fatty tumor has a signal intensity similar to that of subcutaneous fat on the various MR sequences. Some lipomas normally contain internal thin septa of decreased signal. A liposarcoma may contain mature adipose tissue and the diagnosis should be suggested when areas of signal intensity similar to fat are seen within a predominantly soft-tissue mass (7). In lipoblastomatosis, an appearance similar to liposarcoma may evolve during the gradual maturation of a lipoblastoma. Fat-suppression sequences are important. The persistence of high signal in a lipomatous tumor on fat-suppression sequences may be characteristic of lipoblastoma/lipoblastomatosis (13).
The neck is one of the most common locations for a soft-tissue lipoma. On CT and MR studies, this lesion appears as an encapsulated mass. Retropharyngeal lipomas may reach a large size before becoming clinically apparent (11). Fat-containing intraspinal tumors may be focal or diffuse. Focal lesions include lipoma and angiomyolipoma. Lipomas may be intra- or extradural. Intradural lipomas occur around the conus and are associated with lipomyelomeningocele (14). Fat may be seen in a teratoma. Excluding sacrococcygeal teratoma, these neoplasms constitute 0.15% of all intraspinal tumors. Spinal dermoid cysts are usually of mixed signal but may contain areas of signal intensity similar to fat (15, 16). They are more common in the lumbar area, and both teratomas and dermoids may be intramedullary (15) or intradural extramedullary (16). Spinal epidural lipoma is rare in children and occurs in the thoracic area (4). It may rarely cause cord compression. True spinal extradural angiomyolipoma has been described in association with Klippel-Trenaunay-Weber syndrome. The tumor is associated with widening of the intervertebral foramina and displacement of the cord (17).
As well as growing in discrete masses, fatty tissue of the adult type may show diffuse pathologic proliferation of normal extradural unencapsulated fat, a condition known as lipomatosis (4). Diffuse spinal lipomatosis may lead to cord compression. Most cases are associated with corticosteroid administration (18).
Conclusion
Lipoblastomatosis is a tumor that tends to invade locally. Complete excision with a surrounding margin of normal tissue, where possible, is advisable (1, 2). On the other hand, this is a benign tumor and radical cancer surgery should be avoided (3). To our knowledge, this is the first radiologic description of the MR appearance of lipoblastomatosis that includes its maturation after chemotherapy. It is important to recognize the appearance of this neoplasm to avoid confusing it with other, more aggressive, conditions. Plexiform neurofibroma and lymphangioma are important differential diagnoses of immature lipoblastomatosis. The appearance of mature lipoblastomatosis on MR images is almost characteristic.
Footnotes
↵1 Address reprint requests to Dr. Hussein A. M. Kamel, Neuro-Radiology Department, The Royal Victoria Hospital, Grosvenor Rd, Belfast BT12 6BA, United Kingdom.
References
- Received August 5, 1997.
- Copyright © American Society of Neuroradiology