Abstract
Summary: We report a patient with medically refractory mesial temporal lobe epilepsy treated by gamma knife radiosurgery. In lieu of a microsurgical procedure, an entorhinoamygdalohippocampectomy was performed with a gamma knife and low marginal doses (25 Gy). The clinical and imaging studies, including CT, MR imaging, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET), and long-term follow-up MR examinations, are reported. The patient has been seizure-free since the day of treatment, with no clinical complications. MR studies accurately depicted the effect on the target structures and the transient secondary changes around them. FDG-PET scans showed decreased metabolism after gamma knife surgery throughout the anteromesial part of the epileptogenic temporal lobe. This metabolic decrease was reversible in the lateral temporal cortex. Our case suggests that gamma knife surgery is a promising tool for use as a minimally invasive approach to the treatment of epilepsy.
Even if the risk of epilepsy surgery is low, surgical complications may occur, such as postoperative neurologic deficit due to unintended vascular compromise or other accidental damage. To avoid this risk, alternative techniques, such as radiosurgery, may be investigated. Between 1955 and 1973, Talairach and colleagues treated 44 epileptic patients by stereotactic implantation of yttrium-90 into the amygdala and the hippocampus (1). Technical difficulties related to use of the radioelement led to the cessation of this approach, but early clinical results were promising. Some years later, the cessation of seizures after radiosurgical treatment of arteriovenous malformations (AVMs) (2) prompted Linquist et al to use radiosurgery as a way of treating epilepsy (3). Until now, all documented radiosurgical treatment of epilepsy has been in patients with symptomatic, partial epilepsy with space-occupying lesions (3, 4).
We report a patient with partial epilepsy without a space-occupying lesion who was treated by gamma knife surgery in March 1993 with a 5-year follow-up (5). Clinical results and changes documented by MR imaging and positron emission tomography (PET) are described.
Case Report
A 25-year-old man had a 19-year history of drug-resistant complex partial seizures with evidence of a right mesiotemporal lobe origin (infantile febrile convulsions with transitory left hemiparesis, findings at ictal and interictal video EEG monitoring). The mean frequency of complex partial seizures was three per month and the mean frequency of partial seizures with secondary generalization was one per month. The patient underwent bilateral foramen ovale electrode implantation according to the Wieser technique, which demonstrated a unilateral mesiobasal limbic seizure onset: eight seizures started at plot 3 of the right foramen ovale electrode, corresponding to the right amygdala. A Wada test showed no phasic or mnemonic disorder after injection of amytal into the right internal carotid artery. A presurgical MR study showed moderate, diffuse cerebral atrophy with enlarged ventricles and sulci, and a marked focal atrophy of the right hippocampus with slight increased T2-weighted signal intensity throughout the hippocampus. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) showed right-sided interictal hypometabolism that involved the hippocampal region, the temporal pole, and the anterior part of the lateral temporal cortex.
Radiosurgical treatment was performed with a 201-source cobalt-60 gamma unit B model in March 1993 (5). We used MR and CT for target localization and the Kula system for dose planning. The target included the head of the hippocampus and the anterior part of the hippocampal body, the amygdalofugal part of the amygdala, and the entorhinal area. For optimal interpretation of images, the frame was placed in such a way that the base was parallel to that of the temporal horn. The calculated volume of the target was 6440 mm3. According to the volume of the target, we used a marginal dose of 25 Gy at the 50% isodose line. MR imaging and FDG-PET were performed before and regularly after the gamma knife surgery (at 10 and 22 months for PET, and at 3, 8, 10, 11, 12, 13, 16, 19, 24, 25, 31, 37, and 49 months for MR imaging).
The patient has been seizure-free since radiosurgical treatment (at 42 months' follow-up). Antiepileptic treatment has been decreased, but not yet stopped. After a period of diffuse slow waves and sharp waves, predominantly in the right temporal region, EEG recordings became progressively normalized (November 1994). Neurologic examination, including visual field, remained normal. In addition, improvement in behavior was observed by the patient's family. One year after gamma knife surgery, neuropsychological testing showed no changes.
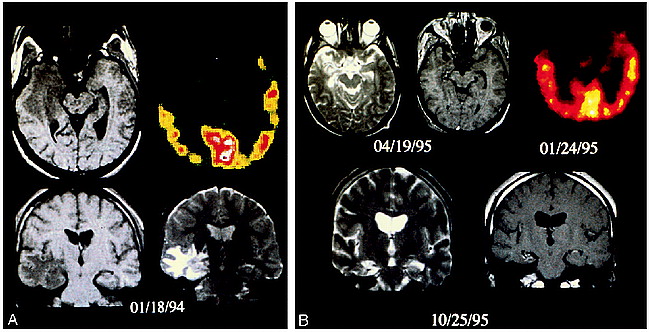
Suddenly, 10 months after surgery (January 1994), radical MR changes appeared; notably, contrast enhancement in the 50% isodose region. Inside the target volume, there was a focally swollen hippocampus with heterogeneous T2-weighted signal hyperintensity (Fig 1A). Outside the target volume, there was a larger area of homogeneously increased T2 signal, which affected predominantly the white matter tracts of the temporal lobe and, to a lesser degree, those of the diencephalon. These findings corresponded to edema in the temporal white matter that also produced a mass effect on the sylvian fissure. On the 10-month FDG-PET study, hippocampal metabolism moderately decreased (−7% vs pretreatment metabolism). Metabolism of the polar region and of the anterior part of the lateral temporal cortex dramatically decreased (−41% and −29%, respectively, vs pretreatment metabolism). No changes were observed in the posterior part of the temporal lateral cortex or in the contralateral temporal lobe.
25-year-old man with 19-year history of drug-resistant complex partial seizures who underwent selective temporomesial gamma knife radiosurgery.
A, Axial T1- and T2-weighted MR images and FDG-PET scan 10 months after treatment show mild right temporal mass effect with effacement of the right perimesencephalic cistern. There is slightly decreased T1 signal and heterogeneous T2 signal in the swollen hippocampus (target). More extensive abnormalities are reflected by diffusely increased T2 signal in the temporal lobe white matter and anatomically continuous tracts, such as the internal capsule, but not in the contiguous but anatomically separate, brain stem.
B, Axial pre- and postcontrast T1- and T2-weighted images 31 months after treatment show diffuse atrophy, a shrunken hippocampus with decreased T1 and increased T2 signal, and minimal surrounding contrast enhancement. The FDG-PET scan shows a decrease in the extent of temporal lobe hypometabolism, now restricted to the medial and polar portion of the lobe.
MR examinations at 24, 25, 31, 37, and 49 months showed only diffuse atrophy, comparable to pretreatment studies, and an encephalomalacic mesial temporal lobe with no more contrastenhancement (Fig 1B).
Discussion
To our knowledge, this is the first reported case of radiosurgical “amygdalohippocampectomy.” The good postsurgical outcome in our patient suggests that gamma knife surgery is an effective technique for treating mesial temporal lobe epilepsy. This noninvasive surgery requires the same treatment criteria as for microsurgical amygdalohippocampectomy, with perfect concordance of all the electroclinical, radiologic, and metabolic data. As previously suggested by Wieser et al for the microsurgical alternative, we consider as a necessity the inclusion of the entorhinal area inside the target volume (6). Our patient had no clinical complications; nevertheless, MR and FDG-PET changes after gamma knife surgery highlighted several issues concerning the safety of this technique.
Morphologic changes observed on the sequential MR images during a 3-year postoperative period provide a relatively unique view of the effects of stereotactic radiosurgery (25 Gy) on “normal” brain (ie, brain without foreign tissue lesions). While there is an extensive literature (see [7] and [8]) on the micro- and macroscopic effects of stereotactic radiosurgery on various animal brains, there is little information on human subjects. Studies in humans have involved patients treated for pathologic lesions, particularly AVMs and tumors. The few reports concerning the effects of stereotactic radiosurgery on normal human brain concern higher dose radioablation of subcortical structures (9–11). Histologically, the lesion consists of coagulative necrosis within the target volume, a thin gliotic rim, and rapid normalization of parenchyma within 1 to 2 mm (7, 8).
In our patient, two modifications appeared after gamma knife surgery: a primary one, looking like radionecrosis of the target, and a secondary one of surrounding vasogenic edema of the white matter. No imaging effects of the radiation were noticed for at least 8 months. At 10 months, an MR examination showed the initially asymptomatic imaging changes. At this time, the target region was swollen with mixed T2 signal, peripheral contrast enhancement, and extensive surrounding white matter T2 hyperintensity. This appearance was consistent with prior reports and suggests early radiation necrosis, adjacent vasculopathy, and surrounding edema or white matter cytotoxic edema secondary to wallerian degeneration (7–9, 12, 13). This pattern evolved over approximately 1 year.
The metabolism of the anteromesial part of the treated temporal lobe decreased after surgery, although the patient was seizure-free. This phenomenon demonstrates that interictal hypometabolism is not directly due to seizures but to other pathophysiological mechanisms.
Although numerous presurgical PET studies of patients with TLE have been performed, the mechanism of temporal hypometabolism remains unclear (14–16). In a previous preoperative FDG-PET study of 22 patients, we described the metabolic pattern of hypometabolism within the temporal lobe of patients with mesial temporal lobe epilepsy (17). This hypometabolism involved the hippocampal region, the temporal pole, and the anterior part of the lateral temporal cortex. We suggested that the main mechanism for temporopolar hypometabolism was deafferentation between the hippocampal area and the temporal pole, and that the mechanism of the lateral temporal cortex might be more complex, involving other factors, such as seizure frequency, seizure propagation mechanisms, and transient postictal changes. To investigate the issue of metabolic changes in nonremoved tissue after selective surgery, Hajek and coworkers studied a series of 12 patients with mesial temporal lobe epilepsy associated with mesial temporal sclerosis who underwent a selective surgical amygdalohippocampectomy and preoperative and postoperative FDG-PET studies (18). These authors reported an ipsilateral decrease of metabolism in the mesial temporal structures, the temporal pole, and the temporal lateral cortex after surgery. Conversely, Dasheiff et al reported that temporal metabolism improved after surgery in a patient with an extratemporal lesion (19). In our patient, the metabolic changes after gamma knife surgery are consistent with the hypothesis of deafferentation for temporopolar hypometabolism, and of neuronal loss for hippocampal hypometabolism. The mechanism of the anterolateral hypometabolism, which increased again after the initial decrease, may be more complex, involving other factors, such as corticocortical connections. The pathophysiological mechanisms of radiosurgery are poorly understood. Sudden-onset CT and MR changes after treatment of large lesions like AVMs with 20 to 25 Gy in peripheral (2, 19, 20) or small volumes of normal brain, with very high doses ranging from 100 to 300 Gy (9–11, 21), are well described. The structural significance of these aspects, called radionecrosis or radioinduced edema, remains unclear. One must also take into account, when studying the appearance of radioinduced neuropathologic changes, the dose, the volume, and the site of radiosurgery. In our patient, a small dose of 25 Gy induced a transient lesion that appeared after 10 months and disappeared within 1 year. In overall appearance and evolution, this lesion complex somewhat resembled that of an infarct, although with a much more protracted time course. This would be consistent with the hypothesis that the mechanism of action of stereotactic radiosurgery is that of primary endothelial damage with secondary vascular obliteration and cerebral infarction. The petechial hemorrhagic signal in the target region and the peripheral contrast enhancement would be expected under this scenario. The secondary vasogenic edema in this case is certainly greater than the usual cerebral infarct. This may related to the protracted time course of the ischemic insult, excess or unique vasotoxins released by the radiated tissue, and/or the additional radiation effects to the surrounding, but not necrosing, cerebral tissue. Against this latter point is the sparing of the brain stem. The mesencephalon, particularly the right cerebral peduncle, would have received the same radiation as the temporal lobe adjacent to the hippocampus, yet the brain stem structures showed no imaging abnormalities. This suggests that it is not the secondary radiation that produced the vasogenic edema but the changes inside the targeted tissue itself.
Vascular physiopathology is probably not the only mechanism in the radiolesion observed. Experimental data have clearly demonstrated the existence of neuronal apoptosis (22). The lesion complex in this patient evolved similarly to those in prior experimental animal reports on stereotactic radiosurgery, but with a longer time frame.
Conclusion
This case demonstrates the ability of gamma knife surgery to successfully treat intractable mesial temporal lobe epilepsy without clinical side effects. The MR studies showed that one can selectively and precisely target a well-defined cortical structure for epilepsy surgery. This is made possible, despite the relatively large size of the target, by the use of lower doses, taking into account the dose volume isoeffective principle. Outside the target area, secondary morphologic changes appeared but were benign and transitory. Late FDG-PET studies showed that the lateral temporal lobe hypometabolism is partly reversible, in contrast to the permanent temporopolar hypometabolism presumed to be secondary to the hippocampopolar deafferentation.
Acknowledgments
We thank G. Kassapian for typing the manuscript.
Footnotes
↵1 Supported by the Assistance Publique des Hôpitaux de Marseille.
↵2 Presented at the annual meeting of the American Epilepsy Society San Francisco, December 1996.
↵3 Address reprint requests to J. Regis, MD, Service de Neurochirurgie Fonctionnelle et Stéréotaxique, Hôpital de la Timone, Boulevard Jean Moulin, 13 008 Marseille, France.
References
- Received August 27, 1997.
- Accepted after revision May 28, 1998.
- Copyright © American Society of Neuroradiology