Abstract
BACKGROUND AND PURPOSE: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is an arteriopathy related to a genetic defect of the notch 3 gene on chromosome 19. The purpose of this study was to evaluate lesion distribution and volume using MR imaging and to correlate the lesion volume with the neurologic and neuropsychological findings.
METHODS: Twenty members of two families (14 with CADASIL as determined by linkage analysis, six healthy) were studied with MR imaging. Two observers evaluated the MR findings semiquantitatively and quantitatively. MR results were then correlated with neurologic and neuropsychological findings.
RESULTS: A typical pattern of lesion distribution in patients with CADASIL was found: the frontal lobe was the site with the highest lesion load, followed by the temporal lobe and the insula. The total lesion volume on T1-weighted MR images correlated significantly with the degree of disability and the degree of impairment in neuropsychological functions (including attention, memory, and conceptual and visuospatial functions).
CONCLUSION: In CADASIL patients, a common pattern of cerebral lesion distribution is found. The total T1 lesion volume is an important parameter to correlate with disability, as it may prove to be helpful in predicting the natural history of the disease.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a recently described syndrome (1–3) that is caused by a genetic defect located on chromosome 19q12 (2) and identified as the notch 3 gene. Clinically, it is characterized by recurrent transient ischemic attacks and strokes, leading to dementia, depression, pseudobulbar palsy, and hemi- or quadriplegia. CADASIL may also be associated with migraine (3). The onset of clinical symptoms usually occurs in the fourth decade (4–6), with a mean age at presentation of 47 years (±10 years) (7). The mean age at death has been reported to be 59 years (±10 years) (7–9).
The syndrome's pathophysiology is still unclear. Genetic evidence for the disease is currently being obtained through linkage analysis (see Appendix), since the defective gene is too large to screen and different point mutations are described within the gene. However, linkage analysis is only possible when large families with several affected members can be investigated. In patients with no known family history, the diagnosis can only be suggested by the presence of typical clinical symptoms and neuroradiologic findings in the absence of arterial hypertension.
In patients from smaller families, an exact description of the pattern of lesions seen on brain MR images could be of significant help in the diagnosis of CADASIL. In addition, correlating the lesion volume with neurologic and neuropsychological findings might help to monitor and improve our understanding of the natural history of the disease. We addressed these issues in a study of two German families in which the diagnosis of CADASIL had been established through genetic linkage analysis.
Methods
Subjects and Genetic Characteristics
Twenty subjects with a mean age of 51 years (range, 31 to 67 years) were studied. Nine subjects were members of one family (family A: four women, five men), and 11 were members of a second family (family B: six women, five men). Asymptomatic relatives were included in the study because of their risk for being disease carriers. Written informed consent was obtained from all subjects before they entered the study.
For genetic linkage analysis, DNA from all family members was obtained and processed according to standard procedures (7). Microsatellite markers that would span the reported CADASIL locus on chromosome 19q12 were used. The numbers and frequencies of alleles used for pairwise and multipoint linkage analysis were chosen from the Genome Data Base (10), and two-point linkage analysis was performed using the FASTLINK implementations of the programs LODSCORE and MLINK (7, 11) (see Appendix). The frequency of the disease gene was 0.0001 and the male and female mutation rate was 0.000001 with equal recombination fractions. Penetrance in heterozygotes was assumed to be 0.96.
Linkage to the reported CADASIL locus on chromosome 19q12 was demonstrated in both families using polymorphic (CA)n repeat markers on chromosome 19p12 (see Appendix). Maximal lodscores of 3.34 (D 19S222 at 𝛉 = 0, family A) and 2.41 (D 19S226 at 𝛉 = 0, family B) were obtained.
Six of the nine members in family A and eight of the 11 members in family B were found to carry the disease haplotype (haplotype and linkage study results of family A have been published previously [7]), and these 14 individuals were considered to be affected by CADASIL. The remaining six family members were considered to be healthy, unaffected, control subjects.
Clinical Assessment
Neurologic Evaluation (n = 20)
All 20 family members underwent a full clinical neurologic examination. Signs of motor, sensory, oculomotor, and central vestibular impairment and symptoms of dysarthria, ataxia, and headache were scored according to the following scale: 0: no impairment, 1: questionable or mild impairment, and 2: definite impairment. In addition, the Expanded Disability Status Scale (EDSS) was used by one of the authors to assess disability in all patients.
Neuropsychological Evaluation (n = 11)
Eleven of the 20 family members (10 with CADASIL, one unaffected) agreed to undergo a neuropsychological evaluation, which was always performed at the same time during the day (eg, 9–11 am). These subjects were assessed with a battery of standardized psychometric tests, as follows:
Intelligence was tested using the German version of the revised Wechsler Adult Intelligence Scale (HAWIE-R) (12).
Conceptual functions were tested using the Wisconsin Card Sorting Test, the Standard and Coloured Progressive Matrices, the Flexibility portion of the “Testbatterie zur Aufmerksamkeitsprüfung” (TAP) (13), and the Picture Arrangement subtest of the HAWIE-R (12).
Attention was tested using the Divided Attention and Incompatibility portions of the TAP (13), the German version of the Trailmaking Test, part A of the “Zahlen-Verbindungs-Test” (14), and the HAWIE-R Digital Symbol subtest (12).
Memory was tested using the Verbal Paired-Associates Learning Test, the Verbal Memory Test (I-S-T 70), the Controlled Oral Word Association Test, and the Benton Visual Retention Test.
Visuospatial functions were tested using the Hooper Visual Organization Test and the Object Assembly and Block Design portions of HAWIE-R (12).
All the test results were rated as follows: 0: no impairment, 1: mild impairment (within 1 SD below mean; SD and mean values being defined for each test by the corresponding publications), and 2: definite impairment (>1 SD below mean). Owing to the differences in each patient's performance, not all subjects completed the whole test battery. The individual extent of impairment was therefore expressed as the ratio between the sum of the domain-specific test results and the number of tests carried out. Thus, the degree of impairment was represented by a continuous scale from 0 (asymptomatic) to 2 (pronounced deficits).
MR Imaging
MR imaging was performed using a 1.5-T or a 1.0-T scanner. Axial T2-weighted double-echo fast spin-echo sequences were obtained with parameters of 2300/14,85/2 (TR/TE/excitations), a section thickness of 5 mm, an intersection gap of 2 mm, a field of view of 230 mm (rectangular), a matrix of 256, and an echo train length of 5. T1-weighted spin-echo sequences were obtained with parameters of 600/15/2, a section thickness of 5 mm, an intersection gap of 2 mm, a field of view of 230 mm (rectangular), and a matrix of 256. Contrast material was used in 12 subjects.
Lesion Load and Lesion Pattern (n = 20)
To determine the distribution pattern of lesions and the lesion load, we evaluated the MR examinations of all 20 subjects semiquantitatively, using a nonlinear scoring system published previously (15). In short, two observers blinded to each subject's clinical status, evaluated the double-echo sequences concordantly, using both echoes (the lesions will be referred to as T2 lesions). Each lesion was then evaluated according to size and site (15). Size was scored as follows: 1: lesion (round or oval) with a diameter (corresponding to the long axis of the lesion) of less than 5 mm; 2: lesion diameter 6 to 10 mm; 3: lesion diameter greater than 10 mm; and 4: confluent lesions (15, 16).
Each lesion was assigned to one of the following sites: a) Subcortical in either the frontal, parietal, temporal, insular, or occipital lobe. The lobe boundaries were identified for all examinations using various landmarks before the evaluation (the borders were marked on the hard copies to ensure that the same borders were used by the second reader). The central sulcus (17, 18), the parietooccipital sulcus, and the temporooccipital incisura were used to separate the various lobes from one another. The insula was identified by its shape and location. Lesions in the limbic lobe were attributed to the closest adjacent lobes (19). b) Corpus callosum. c) Centrum semiovale. d) Periventricular, abutting the lateral ventricles in the region of the cella media, frontal horn, trigone, temporal horn, and occipital horn. e) Basal ganglia: thalamus, caudate nucleus, and lentiform nucleus. f) Internal capsule. and g) Posterior fossa: brain stem and cerebellum.
Quantitative Evaluation (n = 12)
The total lesion volume was determined quantitatively on proton density (PD)—weighted sequences (signal intensity higher than that of the cortex) and T1-weighted sequences (signal intensity lower than that of the cortex) in 11 patients with CADASIL and in one subject not affected by the disease. The remaining eight subjects underwent MR imaging at other institutions; therefore, their electronic data was not available for the quantitative evaluation. In addition, the regional lesion volume was determined using the PD-weighted sequences for each of the five cerebral lobes (representing the subcortical and the periventricular regions of the lesion load evaluation), the basal ganglia, and the posterior fossa. Lesion volume was assessed using a semiautomated local thresholding technique published previously (20).
Statistical Analysis
Owing to methodological differences, we relied on the volumetric data when testing the correlations with the clinical findings, whereas the lesion load data were only used to describe the distribution patterns of the lesions. We used the Spearman rank correlation coefficient (SRCC) to evaluate correlations between lesion volume (PD- and T1-weighted sequences) and neurologic signs in 11 patients with CADASIL and between lesion volume and neuropsychological signs in nine patients with CADASIL. All evaluations were performed using the Statistical Package for Social Sciences.
Results
Clinical Assessment
Neurologic Evaluation (n = 20)
All six unaffected subjects (A1, A4, A5, B1, B6, B9) and one subject with CADASIL (B8) were asymptomatic (Table 1). The most frequent types of impairment in CADASIL patients (n = 14; 13 symptomatic and one asymptomatic) were sensory impairments (10/14 subjects, 71%), followed by hemiparesis (9/14 subjects, 64%) and central vestibular (7/14 subjects, 50%) impairments. Oculomotor symptoms, dysarthria, headache (each in 3/14 subjects, 21%), and ataxia (1/14 subjects, 7%) were found less frequently.
Signs and symptoms of all subjects evaluated
Neuropsychological Evaluation (n = 11)
Of the 11 subjects examined neuropsychologically, two (both with CADASIL) were completely asymptomatic (score, 0) and four (three with CADASIL, one unaffected) had only questionable cognitive impairment (score, <1). The remaining five subjects (all with CADASIL) had mild to clear-cut deficits (score, 1–2) in at least one of the assessed functions (Table 1).
MR Imaging
Lesion Load and Lesion Pattern (n = 20; T2-Weighted)
In three of the six unaffected subjects, no lesions were detected (A5, B1, B9 in Table 2) and in the other three unaffected subjects the lesion load was 16 or less (A1, A4, B6 in Table 2). In the 14 patients with CADASIL, the total lesion load ranged from 29 to 191 (Table 2). Of the 12 patients with CADASIL who had lesion loads between 85 and 191, 10 had fewer lesions, usually large (diameter, >10 mm; score, 3) or confluent (score, 4) (Figs 1 and 2), and the other two patients, including the patient with a score of 191, had a large number of small (lesion diameter, <5 mm; score, 1) lesions (Fig 1A and Table 2). No contrast enhancement was observed in any of the lesions.
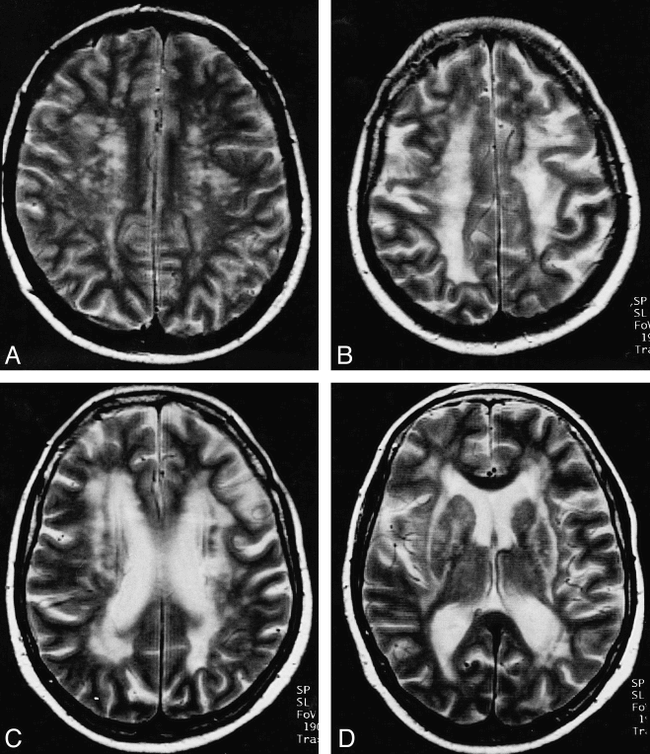
MR lesion load (cerebral white matter changes in T2)
A–D, In the early phase of the disease (subject B3: 35-year-old woman), multiple, small lesions are found in the centrum semiovale (mainly in the frontal lobe) (A). In a later stage (subject B4: 61-year-old woman), confluent lesions are typically found in the white matter of the centrum semiovale (B), the frontal horns and trigones (C), and the internal and external capsule (D)
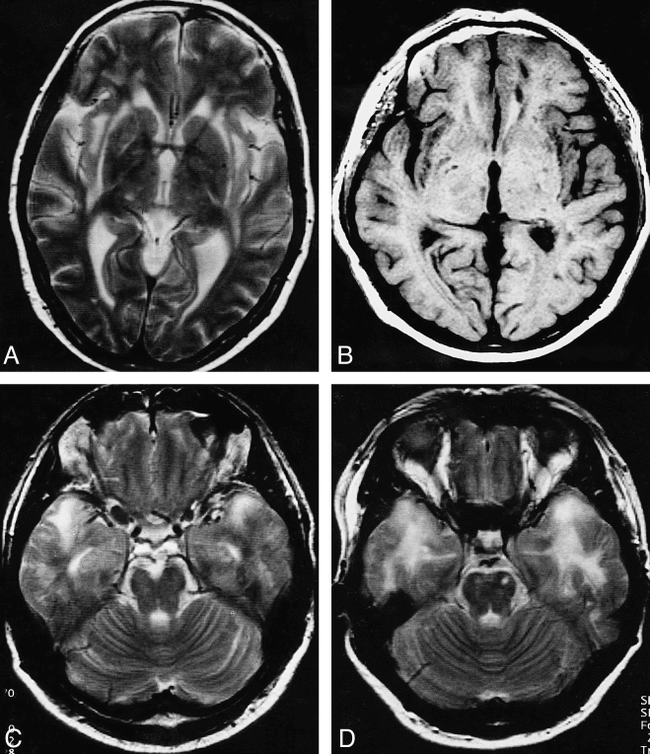
A–D, The typical pattern of lesion distribution includes confluent lesions in the external capsule (A: subject B4), the subcortical white matter of the insula (A and B: subject A2), the subcortical frontal white matter (B), and the temporal poles (C: subject A3, and D: subject A8). The orbital part of the frontal lobe is spared (C)
Areas Affected by Confluent Lesions
Confluent lesions were detected in the brains of 10 of the 14 patients with CADASIL. The distribution of these lesions was as follows: a) Subcortical: frontal (n = 9, bilateral in all, Fig 1B), temporal (n = 8, bilateral in all, predominantly the temporal pole in six patients, Fig 2C and D), insular (n = 9, bilateral in seven, Fig 2A and B), parietal (n = 3, bilateral in all, Fig 1B), and occipital (n = 1, bilateral in all); b) Periventricular: cella media (n = 7, bilateral in all), trigone (n = 7, bilateral in all, Fig 1C), frontal horn (n = 7, bilateral in six, Fig 1C), occipital horn (n = 6, bilateral in all), and temporal horn (n = 4, bilateral in 3); c) Centrum semiovale: (n = 8, bilateral in all, Fig 1B); d) Basal ganglia: (n = 1, caudate nucleus and lentiform nucleus bilaterally); e) Internal capsule: (n = 2, bilateral in both, Fig 1D); f) External capsule: (n = 5, bilateral in 4, Fig 1D); and g) Pons: (n = 1).
Spared Areas
In the 14 patients with CADASIL, the brain area that was least affected by lesions as detected on MR images was the white matter of the basal or orbital part of the frontal lobe. In eight patients this area was not affected at all (Fig 2C, in four patients the area was affected by a single lesion in one hemisphere, and in the remaining two patients the area was affected by one lesion in each hemisphere. The mean lesion load was lowest in the occipital subcortical white matter region (mean score, 0.8). This region was affected in only two of the 14 patients with CADASIL (subjects A8 and B10, Table 2).
Regional Distribution of White Matter Lesions
In the 14 patients with CADASIL, the region with the highest mean lesion load score subcortically was the frontal region (mean, 10.6), followed by the temporal (mean, 6.9) and insular regions (mean, 5.9). The periventricular region with the highest mean lesion load score was the cella media (mean, 10.1), the trigone (mean, 10), the occipital horn (mean, 8.7), and the frontal horn (mean, 8.4) (Table 2). Lesions were almost always detected in individuals with CADASIL in the subcortical frontal (93%, 13/14 subjects; Figs 1A and B, and 2B), temporal (86%, 12/14 subjects; Fig 2C and D), and insular (93%, 13/14 subjects; Fig 2A and B) white matter. Parietal involvement was detected in 79% (11/14 subjects; Fig 1B) of those with CADASIL, and occipital involvement in only 14% (2/14 subjects). Subcortical confluent lesions were mainly found in the frontal (64%, Fig 1B) and temporal (57%, Fig 2C and D) lobes and in the insula (61%, Fig 2A and B); these were seen much less frequently in the parietal (21%, Fig 1B) and occipital (7%, Figs 1 and 2) lobes. Interestingly, the subcortical occipital and frontoorbital regions were clearly less affected than the rest of the occipital and frontal lobes. Periventricular white matter lesions were distributed more or less evenly, with lesions being seen in 71% of patients in the occipital horn and in 100% of patients in the cella media and trigone (Fig 1C). The temporal horn was the least affected by confluent lesions (25%) as compared with the other areas, in which the frequency of confluent lesions ranged from 46% (frontal horn, Fig 1C) to 50% (occipital horn) to 64% (cella media and trigone, Fig 1B). The centrum semiovale was also affected in 79% of cases and contained confluent lesions in 57% of cases (Table 2, Fig 1B).
Quantitative Evaluation of Lesion Volume (n = 12; PD- and T1-Weighted)
Lesion volume was measured in one unaffected subject (B6; age, 54 years) and found to be 1735 mm3 on the PD-weighted sequence and 0 mm3 on the T1-weighted sequence (Table 3). In the 11 patients with CADASIL in whom lesion volume was measured, the mean lesion volume (PD) was highest in the frontal lobe, followed by the parietal and temporal lobes (Table 3). For these patients, total lesion volume measured on the T1-weighted sequence correlated significantly (SRCC = 0.7, P = .03) with that measured on the PD-weighted sequence.
Extended
MR lesion volume (cerebral white matter changes on PD and T1)
Statistical Analysis and Correlation of Neurologic, Neuropsychological, and MR Findings
Control Subjects (n = 6)
The six control (unaffected) subjects (48 to 67 years of age) all had lesion load scores of 16 or less (Table 2), and the one unaffected subject (B6) in whom lesion load was evaluated quantitatively had the lowest measured lesion volume (Table 3). All unaffected subjects were free of neurologic signs and symptoms, and only one (A1; age, 48 years) had a questionable neuropsychological impairment (Table 1).
CADASIL Patients (n = 14)
The two youngest men in our series (B5 and B8; ages 32 and 39 years, respectively) with genetic evidence of CADASIL had only moderate lesion loads (scores = 39 and 29) and lesion volumes (6605 mm3 and 2975 mm3 on the PD-weighted sequence and 0 mm3 on the T1-weighted sequence) and they were, with the exception of headache, neurologically and neuropsychologically asymptomatic. These two men could be categorized as having presymptomatic CADASIL. All the remaining 12 individuals with genetic evidence of CADASIL were found to have intracerebral MR changes as well as neurologic and, in most cases, neuropsychological signs and symptoms of the disorder.
Correlation of Neurologic Findings with Lesion Volume
The degree of disability, as indicated by the EDSS score, correlated significantly with lesion volume (PD) in the parietal lobe (P = .02, r = .7) and basal ganglia (P = .05, r = .6) and with the volume of lesions on T1-weighted images (P = .01, r = .9). The presence of dysarthria correlated significantly with regional lesion volume (PD) in the posterior fossa (P = .03, r = .7), and oculomotor signs correlated significantly with regional lesion volume (PD) in the occipital lobe (P = .02, r = .7).
Correlation of Neuropsychological Impairment with Lesion Volume
The degree of neuropsychological impairment correlated significantly with total lesion volume on T1-weighted sequences: intelligence (P = .05, r = .8), conceptual functions (P = .001, r = .9), attention (P = .006, r = .8), memory (P = .01, r = .8), and visuospatial functions (P = .02, r = .9). Regarding lesion volume on PD-weighted sequences, significant correlations were found between a) intelligence and the insula (P = .03, r = .7); b) conceptual functions and the frontal lobe (P = .005, r = .8), parietal lobe (P = .005, r = .8), insula (P = .005, r = .8), posterior fossa (P = .03, r = .7), and total lesion volume (P = .02, r = .8); c) attention correlated with the parietal lobe (P = .02, r = .8) and the insula (P = .03, r = .9); d) memory correlated with the posterior fossa (P = .01, r = .8); and e) visuospatial functions correlated with the frontal lobe (P = .02, r = .8), parietal lobe (P = .005, r = .8), insula (P = .0001, r = .9), posterior fossa (P = .04, r = .7), and total lesion volume (P = .03, r = .7).
Discussion
In this study we used MR imaging to evaluate the anatomic distribution of lesions affecting the cerebral white matter in patients with CADASIL. We assessed the regional and total lesion load and measured the regional and total lesion volumes, and then correlated these MR findings with neurologic and neuropsychological impairment.
We found that there is a constant pattern of lesion distribution in the brains of patients with CADASIL, with most lesions being seen in the frontal, temporal, and insular lobes; that there is a significant correlation between some of the presenting signs (ie, dysarthria) and the regional lesion volume (ie, posterior fossa) or the total lesion volume; and that T1-weighted lesion volume correlated significantly with disability and neuropsychological impairment and could therefore be of use in monitoring the disease.
From the time that CADASIL was first proposed as a hereditary syndrome (2, 4, 21), neuroimaging has played a vital role in defining the disease. In fact, the characteristic subcortical infarcts and leukoencephalopathies of CADASIL can only be identified in vivo neuroradiologically, using either CT or MR imaging. Neuroimaging was therefore not only essential in the initial description of CADASIL but has become indispensable in the diagnosis of this syndrome (9, 22).
Pattern of Cerebral Involvement
In the present study, the majority of symptomatic CADASIL patients (n = 12/14) were found to have extensive involvement of the subcortical and periventricular white matter, with clear regional differences. The typical pattern of cerebral changes in individuals with CADASIL can be summarized as follows: 1) symmetric involvement by confluent lesions located subcortically in the frontal and temporal (temporal pole) lobes as well as in the insula, periventricularly (lateral ventricles), in the centrum semiovale, and in the internal and external capsule, basal ganglia, and brain stem; and 2) relative sparing of the frontoorbital and the occipital subcortical regions.
Previous imaging and postmortem studies have shown that affected white matter is located in the frontal (5), frontal and temporal (6), frontal and parietal (23), and periventricular (3, 5, 6) areas. Other reports have documented involvement of the basal ganglia, including the thalamus, as well as the brain stem and cerebellum (3–6, 21, 24, 25). Some have identified frequent involvement of the external and internal capsule (3, 4, 9, 24, 26, 27) and others (3) have noted that the external capsule, basal ganglia, and brain stem are spared. The MR findings in studies correlating CADASIL with familial hemiplegic migraine (assessed qualitatively) were similar to those previously described, although a predilection for the anterior temporal and the occipital periventricular white matter was found (22, 28).
In general, these descriptions are in line with the results of our analyses. In addition, we found lesions in insular subcortical white matter (Fig 2A and B) (one report described involvement of the insular cortex in two patients [21]) and relative sparing of the orbital frontal lobe (Fig 2C) and the subcortical occipital white matter.
Histopathologic studies have shown a characteristic angiopathy of small and middle-sized arteries to be the underlying cause of CADASIL. The angiopathy involves duplication and splitting of the internal elastic lamina, hypertrophy of the media, and adventitial hyalinosis and fibrosis (1, 3, 6). Basophilic granular material replaces or destroys the smooth muscle cells of the media, although there are no appreciable atherosclerotic changes or amyloid depositions, and eosinophilic granular deposits have been noted in the media, not confined to the muscular cell layer but extending into the adventitia in the form of coarse, occasionally coalescent, masses (3). The cerebral arteries affected most are those supplying the white matter, basal ganglia, thalamus, leptomeninges, cerebral cortex, and cerebellum (25), which are the areas in which we identified high lesion load and volumes on MR images.
Confirming a suspected diagnosis of CADASIL can be challenging, especially in individuals from small families, because genetic analysis is of limited value. On the other hand, there is increasing evidence that CADASIL is not as uncommon as was previously thought (3), which increases the importance of determining what specific pattern of lesions on MR images may be correlated with a diagnosis of CADASIL versus other diseases affecting the white matter, in particular, subcortical arteriopathic encephalopathy (SAE). In this latter case, the patient's age could be of help, because CADASIL tends to be diagnosed when patients are in the fourth to fifth decade, in contrast to SAE, which is diagnosed at a later age (sixth to seventh decade). It will still be necessary to evaluate the specificity of the described pattern of MR changes in CADASIL with those occurring in other white matter—affecting diseases, such as SAE; mitochondrial myopathy encephalopathy, lactic acidosis, and strokelike episodes (MELAS); and vasculitis.
Correlation of Clinical Findings with Lesion Volume
The results of previous studies that investigated the correlation between neuroradiologic changes and neurologic and neuropsychological impairment in patients with CADASIL have been conflicting. It is known that white matter changes in presymptomatic CADASIL patients are moderate as compared with the extensive changes seen in symptomatic patients. Nevertheless, some studies have found a lack of correlation between imaging and clinical findings (25).
Our results, in contrast, show a significant relationship between clinical findings and lesion volume. The significant correlation of higher EDSS scores with the presence of lesions in the basal ganglia (PD), internal capsule T2), brain stem (T2), and cerebellum (T2) is in line with what is known about the functional anatomy of these areas. The same is true for the correlation between the presence of dysarthria and a high lesion volume in the posterior fossa (PD). Some other correlations in our study cannot be explained by functional anatomy. At least two explanations are possible for these findings. First, the relatively small number of subjects we examined and the large number of possible correlations could have limited the power of our statistical evaluation. Second, the increase in lesion volume in areas known not to be related to the affected function could have been incidental, occurring as the total lesion volume increased.
A constant finding in our study was the significant correlation between the total lesion volume as determined on T1-weighted sequences and the degree of disability (EDSS) and impairment of intelligence, attention, memory, conceptual and visuospatial functions.
Significance of Lesion Type
Two types of lesions have been described in patients with CADASIL (4, 9, 21, 23, 25, 28). The first type is a large, sometimes coalescent lesion located in the white matter that is hyperintense on T2- and PD-weighted sequences and isointense on T1-weighted sequences. The second type is a small, well-delineated lesion that spares the cortex and that also is hyperintense on T2- and PD-weighted images but is hypointense on T1-weighted sequences.
Histopathologic studies of brains from CADASIL patients have revealed the presence of diffuse white matter pallor with some preservation of the U fibers (1, 23, 29). In addition, small necrotic foci have been found scattered throughout the white matter, showing all degrees of destruction from a spongy looseness of the tissue with loss of myelin sheath to typical small (3- to 6-mm) cystic infarcts (1, 3, 6, 23, 25, 29). It can be assumed that both types of lesions would have appeared hyperintense on T2- and PD-weighted sequences, whereas infarcts would have appeared hypointense on T1-weighted sequences.
As already noted, the degree of neuropsychological impairment and the degree of disability (as measured by the EDSS) always correlated significantly with the T1 lesion volume in patients with CADASIL in this study. The fact that the volume of lesions identified on T1-weighted MR images has been found to correlate closely with disability in two such different diseases as multiple sclerosis and CADASIL underlines the impact that T1-weighted lesions have on patient functioning, regardless of whether the lesions result from an inflammatory or avascular process (30, 31). In contrast, lesions identified on T2-weighted images can represent edema, gliosis, or demyelination rather than tissue destruction, and therefore might not always be clinically significant.
Conclusion
Dementia has been reported to be one of the main symptoms of CADASIL (3, 4, 21), and it is such a prominent symptom that the syndrome was previously named hereditary multiinfarct dementia (5, 6). Although dementia may be the first symptom (5), it is usually observed in a late stage of the disease (25). The correlation between neuropsychological impairment and lesion volume as determined on T1-weighted sequences (in contrast to the limited correlation with lesion volume on PD-weighted sequences) is in line with the late occurrence of dementia, at a stage when extensive T2 lesions exist and T1 lesions appear. In our study, as well as in others (24), asymptomatic subjects were found to have lesions on T2-weighted sequences but not on T1-weighted sequences. T1 lesion volume could therefore play an important role in monitoring the natural history of CADASIL or the efficacy of a possible therapeutic regimen.
Appendix
Linkage analysis: This method is based on the observation that 1) neighboring genetic loci are usually transmitted together and that 2) the probability of cotransmission/separation during meiosis is dependant on the genetic distance between the two loci.
Genome Data Base: A data base that carries detailed information on large parts of the human genome (genes, genetic segments, and other genetic data). The data base is regularly upgraded and accessible via the Internet.
Lodscore: A measure for the likelihood of linkage (logarithm of the odds). By convention, a lodscore of 3 is accepted as sufficient to demonstrate linkage. For secondary linkage, a lodscore of 2 is required.
FASTLINK, LODSCORE, MLINK: Computer programs used for genetic linkage analysis.
Polymorphic (CA)n repeat markers: Markers used to molecularly characterize polymorphic genetic loci.
Disease haplotype: Haplotype carrying the disease causing a genetic defect as opposed to the unaffected “healthy” haplotype.
Acknowledgments
The Dispim image display program was written and provided by D. Plummer (Department of Medical Physics, University College, London, UK). The program converting the MR data into a format readable for lesion load measurement was written and provided by M. A. Horsfield (Department of Medical Physics, University of Leicester, Leicester, UK). We thank U. D. Schmid (Neurosurgical Unit, Klinik Im Park, CH-8027 Zürich, Switzerland) for revising the manuscript and D. Mathis (Professional Communications Consultant, Pittsburgh, PA) for editing the manuscript.
Footnotes
↵1 Supported by grants from the Friedrich-Bauer-Foundation Munich (to T.A.Y. and U.D.S.), the Weigand-Foundation Munich (to T.A.Y.), and the Münchner Medizinische Wochenschrift (to T.A.Y.).
↵2 Presented at the annual meeting of the American Society of Neuroradiology, Toronto, Ontario, May 1997.
↵3 Address reprint requests to Priv. Doz. Dr. med. Tarek A. Yousry, Department of Neuroradiology, Klinikum Grosshadern, Marchioninistr. 15, D-81377 Munich, Germany.
References
- Received March 19, 1998.
- Copyright © American Society of Neuroradiology