Abstract
BACKGROUND AND PURPOSE: Functional reorganization of the brain can result from congenital brain disorders as well as from brain infarction. The purpose of our study was to use functional MR imaging to determine whether reorganization of brain function occurs in patients with schizencephaly.
METHODS: Four patients with schizencephaly (three right-handed, one ambidextrous) presented with seizures. Associated lesions included agenesis of the corpus callosum (n = 1) and absence of the septum pellucidum (n = 1). Functional MR imaging was performed in each patient using a single-section fast low-angle shot (FLASH) blood oxygen level-dependent (BOLD) technique at 1.5 T in a standard head coil. The motor cortex was initially identified on an axial T1-weighted anatomic image. Thirty consecutive images were obtained during a motor task consisting of repetitive finger-to-thumb opposition. The percentage of change in increased signal intensity was calculated for the primary motor area. An ipsilateral activation index was used to compare the affected with the unaffected hemisphere.
RESULTS: The percentage of change in increased signal intensity in the area of activation ranged from 4.8% ± 0.9 to 9.2% ± 1.2 (mean, 5.6% ± 1.5). The ipsilateral activation index in the affected hemisphere was 0.00 to 0.38, whereas that in the unaffected hemisphere was 15.4 to infinity. The difference in the ipsilateral activation index between each hemisphere was considered significant.
CONCLUSION: Our results showed increased activation in the unaffected hemisphere in patients with schizencephaly, which may reflect functional reorganization of the motor area in patients with this congenital disorder.
Functional MR imaging can be used to demonstrate reorganization of the primary motor area in patients with brain tumor (1). The location of the functioning cortex may be altered by plasticity of the brain, a process by which neurons in normal brain take over the function of damaged or diseased brain caused by an acquired lesion, such as an infarction or brain tumor (1–5). Even in congenital brain lesions, this process will occur. The purpose of this study was to use functional MR imaging to identify possible reorganization of brain function in patients with congenital schizencephaly.
Methods
Four patients with schizencephaly (three men, one woman; three right-handed, one ambidextrous; mean age, 23.5 years) presented with seizures. Associated lesions included agenesis of the corpus callosum (n = 1) and absence of the septum pellucidum (n = 1). MR imaging was performed using a single-section fast low-angle shot (FLASH) blood oxygen level-dependent (BOLD) technique on a 1.5-T imaging system with a standard head coil. T1-weighted anatomic images were obtained in the oblique axial plane, in which the primary motor cortex was located in the middle of the field of view. Parameters for the FLASH sequence were as follows: 90/56 (TR/TE); flip angle, 40° matrix, 56 or 64 × 128; field of view, 20 × 20 cm, section thickness, 9 mm; acquisition time, 7 or 8 seconds for each image with the same field of view and section thickness as in the corresponding anatomic images. All 30 consecutive images were obtained in one sequence.
The motor task consisted of repetitive finger-to-thumb opposition movements of each hand (2 Hz). One study included three sets consisting of alternating active and rest periods. Five images were obtained in each period. Activation images were obtained by subtracting sets of control images from those acquired during activation. To ensure that the images reflected steady-state conditions, the first image in each block of five was omitted from the additions and subtractions. Registration of the activation and anatomic images was achieved by a pixel-to-pixel identification of key features in the anatomic, control, and activation images (6). Activation pixels were superimposed on the anatomic template. Signal intensities and activations were presented as means ± standard deviation. Statistics included cross-correlation analysis. The threshold was set at 0.7, for which the P value was calculated at .0001. The percentage of change in increased signal intensity was calculated for the primary motor area. We counted only the activated pixels clustering in the area anterior to the central sulcus. The central sulcus was identified by anatomic criteria (7). The ratio of ipsilateral to contralateral activation was calculated as follows: ipsilateral activation index = number of activated pixels in the ipsilateral motor area/number of activated pixels in the contralateral motor area (1).
Results
All procedures were successfully carried out in the four patients, all of whom had contralateral hemiparesis (mild in three, moderate in one). With a view toward optimizing the signal changes observed with cortical activation, data were obtained from four subjects for each motor task using both the right and left hands. The percentage of change in increased signal intensity in the activated area ranged from 4.8% ± 0.9 to 9.2% ± 1.2 of baseline (mean, 5.6% ± 1.5). The activated pixels were generally clustered. Typically, the activated area in the ipsilateral motor area was much smaller than that in the contralateral motor area. All subjects had a much larger activation area of the motor strip in the unaffected hemisphere than in the affected hemisphere (see Table). The ipsilateral activation index in the affected hemisphere was 0.00 to 0.38, but that in the unaffected hemisphere was 15.4 to infinity. A significant difference was found in the ipsilateral activation index between the two hemispheres (paired Student t-test, P < .01). In patient 1 (Fig 1), who had a high grade of cerebral deformity of the right hemisphere, motor activation occurred in the contralateral hemisphere despite the presence of a mild cortical dysplasia on the left. In three patients, the activated areas were consistent with finger movement involving the precentral gyrus that would be expected from activation by this task; in patient 1, the activation from finger movement was widespread and lower than that which would be expected in normal brain. The functional MR imaging of the other three patients showed a normal activation pattern and localized activation of the hand-motor task in the unaffected hemisphere (Fig 2).
Results of neurologic symptoms and functional MR imaging in schizencephaly
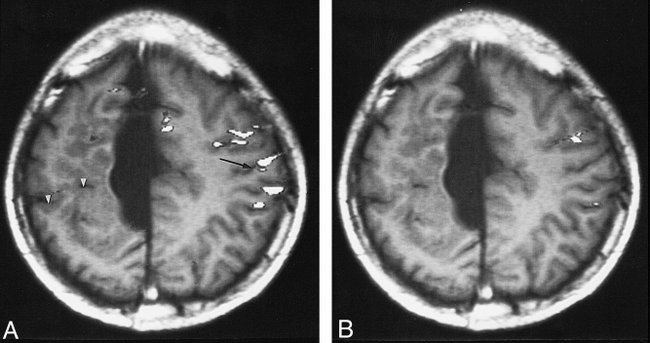
Patient 1, with schizencephaly and agenesis of the corpus callosum and an associated interhemispheric cyst. The ipsilateral activation index on the unaffected hemisphere is infinite.
A, Functional MR image (90/56; T1-weighted image [300/14/2]) shows the widespread and lower-lying activation areas adjacent to the central sulcus (arrow) during the right-hand motor task. Arrowheads indicate schizencephaly.
B, Functional MR image (90/56; T1-weighted image [300/14/2]) shows the activation area of the left-hand motor task located in the less-affected hemisphere.
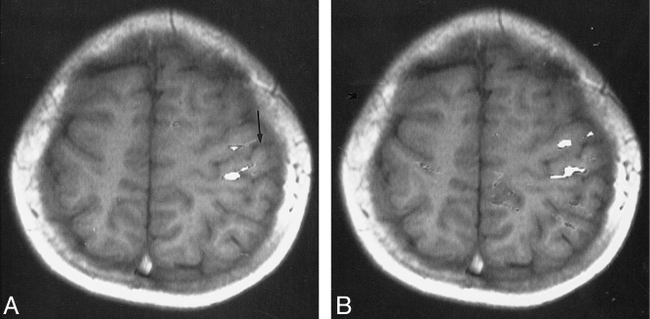
Patient 3, with schizencephaly and focal cortical dysplasia. Abnormal gyral pattern is noted in the right hemisphere. Ipsilateral activation index on the unaffected hemisphere is 15.4.
A and B, Functional MR images (90/56; T1-weighted images [250/14/2]) show the activation areas located in the vicinity of the central sulcus of the unaffected hemisphere during the right-hand (arrow, A) and left-hand (B) motor tasks.
Discussion
In previous studies, functional MR imaging has been used to locate the rolandic area (1, 8). The accuracy of this technique in locating the primary sensorimotor cortex has been verified by comparison with intraoperative mapping techniques (9, 10).
The anatomic location of function may be altered by plasticity, in which neurons in normal regions of the brain take over for the function of damaged or diseased brain. Several reports have described the reorganization of the functional cortex in acquired brain disorders using positron emission tomography (2, 3), magnetoencephalography (4), and electromyographic recording (5), but not in congenital brain disorders. Yoshiura et al (1) found increased activity in the contralateral motor area on functional MR images obtained during a hand-motor task in patients with brain tumor. Our findings also revealed decreased activity in the affected hemisphere and increased activation in the unaffected hemisphere. A possible explanation for the high activation index in the unaffected hemisphere is the decreased brain function on the affected side caused by the deformed brain, suggesting that most of the functioning corticospinal tract fibers arise not from the affected hemisphere but from the unaffected hemisphere.
The findings obtained with the BOLD technique may be explained primarily by changes in blood oxygenation; in particular, a net conversion of deoxyhemoglobin to oxyhemoglobin. These changes are confined to gray matter, presumably because the hemodynamic changes are also confined to gray matter; therefore, in any modeling of the observed effects, one must consider blood vessels and hemodynamic changes that are specifically related to the activated gray matter regions (11).
Behavioral studies show some recovery of basic sensory and motor functions after cortical injury, particularly if the injury is sustained in infancy or early childhood. Motor and sensory functions may not be uniquely located in the rolandic cortex, as some current thinking suggests (5). One report found that locating the rolandic cortex by anatomic landmarks using MR imaging was unreliable in 16% of healthy subjects and in 35% of patients with lesions (7). With our patient 1, motor activation for hand movement was detected at a lower level than that expected for normal brain. For identifying the central sulcus, landmarks can be identified on axial images, midline sagittal images, or parasagittal images. In axial images, the superior frontal sulcus, precentral sulcus, and superior genu of the central sulcus are considered reliable landmarks for locating the rolandic cortex in healthy subjects. However, tracing the central sulcus through a series of images can be difficult in patients with severe migration disorders, such as schizencephaly, owing to the presence of a deformed gyral pattern. In our cases, identification of the central sulcus of the affected hemisphere was difficult. For this reason, we obtained lower or higher axial sections in addition to the sections from the functioning area of hand movement to confirm the primary motor area.
Barkovich (12) found that the abnormal venous drainage is paralleled by the congenital deformity of the brain cortex. In our study, patient 1, with associated focal cortical dysplasia in the less-affected hemisphere, had an unusual pattern of widespread and lower-lying activation areas of finger movements on the same side, which may have been caused partly by the abnormal course of the cortical venules and capillary network and partly by the functioning reorganization of motor function of the less-affected hemisphere.
In our study, we used a single-section FLASH technique, whereas most functional MR imaging involves use of echo planar imaging or magnetic fields of 2 T and above to obtain better BOLD effects. The FLASH technique has greater in-flow effect and is time-consuming as compared with echo planar imaging (6). Although the in-flow effect hinders the localization of exact functioning areas, it did not lead to any erroneous interpretation in our study.
Conclusion
Functional MR imaging of our patients with schizencephaly showed a much larger activation area of the motor strip in the unaffected hemisphere than that in the affected hemisphere. It may reflect a reorganization of functioning brain during a hand-motor task to the unaffected or less-affected hemisphere.
Footnotes
↵1 Address reprint requests to Ho Kyu Lee, MD, Department of Diagnostic Radiology, Asan Medical Center, University of Ulsan, 388-1 Pungnap-dong, Songpa-ku, Seoul 138-736, Korea.
References
- Received January 22, 1998.
- Copyright © American Society of Neuroradiology