Abstract
BACKGROUND AND PURPOSE: Transoral robotic surgery is an emerging strategy for treating human papillomavirus–positive cancers, but the role of MR imaging in predicting the surgical outcome has not been established. We aimed to identify preoperative MR imaging characteristics that predispose the outcome of transoral robotic surgery toward an insecure (positive or close) surgical margin in human papillomavirus–positive tonsillar squamous cell carcinoma.
MATERIALS AND METHODS: Between December 2012 and May 2019, sixty-nine patients underwent transoral robotic surgery at our institution. Among these, 29 who were diagnosed with human papillomavirus–positive tonsillar squamous cell carcinoma, did not receive neoadjuvant treatment, underwent preoperative 3T MR imaging, and had postoperative pathologic reports and were included in this retrospective study. Two neuroradiologists evaluated the preoperative MR imaging scans to determine the tumor spread through the pharyngeal constrictor muscle using a 5-point scale: 1, normal constrictor; 2, bulging constrictor; 3, thinning constrictor; 4, obscured constrictor; and 5, tumor protrusion into the parapharyngeal fat. The risk of an insecure surgical margin (involved or <1 mm) according to the MR imaging scores was predicted using logistic regression with the Firth correction.
RESULTS: The interobserver agreement for the MR imaging scores was excellent (κ = 0.955, P < .001). A score of ≥4 could predict an insecure margin with 87.5% sensitivity and 92.3% specificity (area under the curve = 0.899) and was the only significant factor associated with an insecure margin in the multivariable analysis (OR, 6.59; 95% CI, 3.11–22.28; P < .001).
CONCLUSIONS: The pre-transoral robotic surgery MR imaging scoring system for the pharyngeal constrictor muscle is a promising predictor of the surgical margin in human papillomavirus–positive tonsillar squamous cell carcinoma.
ABBREVIATIONS:
- AUC
- area under the curve
- cN
- clinical node
- cT
- clinical tumor
- HPV
- human papillomavirus
- pN
- pathologic node
- pT
- pathologic tumor
- ROC
- receiver operating characteristic
- SCC
- squamous cell carcinoma
- TORS
- transoral robotic surgery
Oropharyngeal squamous cell carcinoma (SCC) is a head and neck cancer with increasing prevalence as a consequence of rising human papillomavirus (HPV) infections.1,2 HPV-positive oropharyngeal SCC is known for its excellent prognosis with substantially improved survival compared with HPV-negative SCC.3⇓-5 Surgery, radiation, and chemotherapy are the main treatment methods for oropharyngeal SCC and can be used alone or in combination depending on the cancer stage.6
The treatment protocol for HPV-positive SCC has shifted toward a “deintensification” approach to maintain favorable oncologic outcomes while minimizing treatment-related morbidity.7⇓-9 Long-term adverse effects from radiation or chemotherapy and high morbidity from traditional surgery through external mandibulotomy can reduce the quality of life, particularly in young patients who have to live with the consequences for far longer.10⇓⇓–13 Recently, transoral robotic surgery (TORS) has emerged as a first-line treatment, particularly for early-stage HPV-positive oropharyngeal SCC.14,15 While avoiding functional deficits from the traditional external approaches, TORS can reduce the need for adjuvant therapy after surgery or can use surgery as a single-technique therapy while preserving oncologic outcomes, particularly when the negative margin is achieved by TORS.2,3,5,16⇓-18
Despite these advantages, there is still a risk of obtaining an insecure surgical margin (ie, positive margin involvement by the tumor or a close margin of <1 mm between the tumor and the margin) in TORS, which necessitates adjuvant therapies, even in early T1 and T2 tumors.3,19 Because oncologic outcomes in such cases are similar to those with chemoradiation alone,3 it is important to preselect patients who are expected to have an insecure surgical margin to avoid unnecessary dual treatment. However, no published study has evaluated the preoperative MR imaging characteristics that can predict the surgical margin after TORS. It has been noted that tumor invasion through the pharyngeal constrictor muscle confirmed in a surgical field will likely have a positive margin related to locoregional recurrence, but data supporting an imaging-based predictor are still lacking.2,5,20
In our study, we aimed to identify preoperative MR imaging characteristics, particularly with regard to pharyngeal constrictor muscle involvement by the tumor in early stage cancers, that predispose the outcome of TORS toward an insecure surgical margin in HPV-positive tonsillar SCC.
MATERIALS AND METHODS
Study Subjects
This retrospective study was approved by our institutional review board (B-1906-544-101), and the requirement for written informed consent was waived. Between December 2012 and May 2019 at our institution, a nationwide third-referral hospital, we included 36 subjects who met the following criteria: 1) diagnosed with HPV-positive tonsillar SCC, 2) had preoperative 3T MR imaging available, and 3) had postoperative pathologic reports available. Among them, 6 who received neoadjuvant therapy and 1 who did not have a postoperative pathologic report available were excluded from the analysis.
Clinical records were examined for demographic characteristics and staging, and treatment data were obtained from electronic medical records. The clinical primary tumor (cT) and nodal (cN) categories were staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual.21 All included subjects underwent TORS using the da Vinci surgical robotic system (Intuitive Surgical) performed by 2 head and neck surgeons (W-J.J. and Y.H.J. with 10 and 15 years’ experience in head and neck surgery, respectively). Adjuvant treatment was considered when the cancer-free margin was insufficient. Follow-up surveillance was initially performed 3 months after the operation and every 3–6 months after the initial follow-up using CT or MR imaging and/or PET/CT to assess locoregional recurrence. The treatment option for each patient was decided at a weekly multidisciplinary tumor board including otorhinolaryngology–head and neck surgeons, oncologists, radiologists, and pathologists, following the 2018 National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for oropharyngeal cancer.22
Image Acquisition
MR images were acquired using a 3T MR imaging unit (Ingenia; Philips Healthcare) with a 32-channel sensitivity encoding head coil. Axial TSE T2WI was performed with and without fat suppression using the multipoint Dixon technique. The imaging parameters were as follows: TR, 3300 ms; TE, 80 ms; FOV, 180 × 220 mm2; acquisition matrix, 440 × 440; section thickness, 3 mm; no section gap; NEX, 1. Other parameters for the full MR imaging sequences are described in the On-line Appendix.
Image Analysis
The cT category according to the 8th edition of the AJCC Cancer Staging Manual for the primary tumor was verified by 1 board-certified neuroradiologist (Y.J.B. with 10 years’ experience in neurology and head and neck imaging) on the basis of MR imaging.
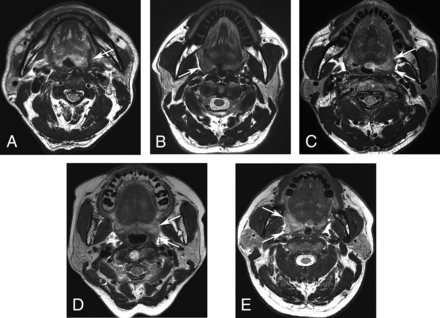
On axial T2WI, tumor spread through the pharyngeal constrictor muscle was independently determined by 2 board-certified neuroradiologists (Y.J.B. and B.S.C. with 20 years’ experience in neurology and head and neck imaging) who were blinded to the clinical and histopathologic information. The following 5-point scale scoring system was used for the assessment of the status of the pharyngeal constrictor muscle: 1, normal constrictor; 2, bulging constrictor; 3, thinning constrictor; 4, obscured constrictor; 5, tumor protrusion into the parapharyngeal fat (Fig 1). After an independent reading, the final MR imaging score was designated by 2 readers in consensus and used for further analysis.
Representative 5-point scale preoperative MR imaging scores for tumor spread through the pharyngeal constrictor muscle. A, Axial T2WI of a 79-year-old man with left-tonsillar SCC revealing a normal constrictor muscle (arrow) (score 1). B, Axial T2WI of a 59-year-old man with right-tonsillar SCC revealing the bulging contour of the constrictor muscle due to the tumor, but normal thickness (arrow) (score 2). C, Axial T2WI of a 66-year-old man with left-tonsillar SCC reveals thinning of the constrictor muscle due to the tumor (score 3). Note that the thickness of the left constrictor muscle is reduced compared with the right side (arrows). D, Axial T2WI of a 69-year-old man with left tonsillar SCC revealing the obscured margin of the constrictor muscle by the tumor (score 4). Note that the normal contour of the constrictor muscle is not visualized (arrows). E, Axial T2WI of a 54-year-old man with right-tonsillar SCC revealing definite protrusion of the tumor into the parapharyngeal fat (arrows) (score 5).
Histopathologic Review
According to the 8th edition of the AJCC Cancer Staging Manual,21 the pathologic tumor (pT) and the nodal (pN) statuses were staged by 1 pathologist (H.K.) who specialized in head and neck pathology with 11 years’ experience. First, in pathologic specimens, the histologic pharyngeal constrictor muscle invasion by the tumor was evaluated and determined as negative or positive. Next, the status of the surgical margin was determined using the excised superior constrictor muscle as the deep margin. If the surgical margin involved the tumor, it was defined as a positive margin. A close margin was defined as a distance between the surgical margin and the tumor of <1 mm. Positive and close margins were designated as insecure surgical margins. A negative margin was defined as a distance between the surgical margin and the tumor of ≥1 mm.
Statistical Analysis
Continuous variables are expressed as the median and range. Clinico-histopathologic findings according to the final surgical margin status were compared using the Fisher exact test and the Mann-Whitney U test. Interobserver agreement of MR imaging scores between the 2 readers was tested by Cohen κ coefficient statistics: >0.75, excellent agreement; 0.40–0.75, fair-to-good agreement; and <0.40, poor agreement.23 The relationship between the MR imaging score and the histologic pharyngeal constrictor muscle invasion by the tumor was tested using the Linear-By-Linear Association test and the Spearman correlation. Diagnostic performances predicting an insecure surgical margin using MR imaging scores were evaluated using receiver operating characteristic (ROC) curve analysis. Area under the curve (AUC) values from each ROC curve analysis were compared using the DeLong test.24 We further used the univariable and multivariable logistic regression with a Firth correction25 for risk evaluation of MR imaging scores for the pharyngeal constrictor muscle status to predict an insecure surgical margin after TORS. P values < .05 were considered statistically significant. Statistical analyses were performed using SPSS software (Version 17.0; IBM), MedCalc 17.9 (MedCalc Software), and SAS (Version 9.3; SAS Institute).
RESULTS
Clinico-Histopathologic Findings According to the Surgical Margin
According to the inclusion and exclusion criteria, 29 patients (26 men, 3 women; age range, 38–80 years; mean age, 61.6 years) were included for further analysis. Baseline patient characteristics are presented in Table 1. The cT categories were primarily T2 (n = 23), followed by T1 (n = 5), and T4 (n = 1). Twenty-seven patients underwent TORS with nodal dissection, and 2 patients underwent TORS alone without nodal dissection. The histopathologic results revealed a positive surgical margin in 7 patients, including 1 patient with cT4 who did not undergo neoadjuvant therapy, and a close surgical margin in 9 patients, for a total of 16 patients with an insecure surgical margin. After TORS, 18 patients underwent adjuvant chemoradiation (n = 10) or radiation only (n = 8), including 11 patients with an insecure surgical margin. Thirteen patients, including 8 with an insecure surgical margin, had lymphovascular invasion, and 2 patients with an insecure surgical margin had perineural invasion in the surgical specimen. There were no differences in the clinico-histopathologic findings according to the surgical margin status (Table 1). On-line Table 1 summarizes the patient information regarding the tumor stage, MR imaging and pathologic findings, and adjuvant therapy.
Clinico-histopathologic findings according to the surgical margin status
Interobserver Agreement of MR Imaging Scores for Pharyngeal Constrictor Muscle Involvement
The interobserver agreement between the 2 readers was excellent (κ = 0.955; P < .001). The final MR imaging scores were 1 in 5 patients, 2 in 4 patients, 3 in 5 patients, 4 in 9 patients, and 5 in 6 patients.
Correlation between the Preoperative MR Imaging Score and Histologic Pharyngeal Constrictor Muscle Invasion
There was a significant difference in the state of histologic pharyngeal constrictor muscle invasion by the tumor according to the MR imaging scores (On-line Table 2). Patients with higher MR imaging scores showed a trend toward a positive histologic pharyngeal constrictor muscle invasion by the tumor (Spearman correlation coefficient, 0.601; P = .001).
Diagnostic performance of the MR imaging score for predicting an insecure surgical margin
Prediction of the Surgical Margin Using the Preoperative MR Imaging Scores
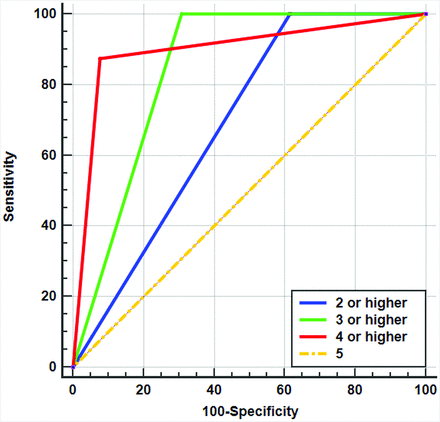
In the ROC analysis, MR imaging scores of ≥4 (AUC = 0.899; 95% CI, 0.730–0.979) and ≥3 (AUC = 0.846; 95% CI, 0.664–0.952) enabled good prediction of an insecure surgical margin (Fig 2). However, there was no difference in the diagnostic performance between the 2 thresholds (P = .477). Optimal cutoff scores with AUCs, sensitivities, and specificities are provided in Table 2.
ROC curves for diagnosing an insecure surgical margin according to MR imaging scores for the pharyngeal constrictor muscle involvement. The ROC curve of the MR imaging score of ≥4 (red) has the highest AUC value of 0.899, followed by the MR imaging score of ≥3 (green, AUC value of 0.846), MR imaging score of ≥2 (blue, AUC value of 0.692), and MR imaging score of 5 (orange, AUC value of 0.500).
The results of the univariable analysis of the clinico-histopathologic factors and the MR imaging scores affecting the surgical margin status are presented in Table 3. The MR imaging scores were the most significant predictive factors of an insecure margin (P < .05). In the multivariable analysis of the variables with P values < .15 in the univariable analysis (ie, cT, pT, and MR imaging score), an MR imaging score of ≥4 was the only significant predictive factor of an insecure surgical margin (Table 4).
Univariable analysis of predictors of an insecure surgical margin
Multivariable analysis of predictors of an insecure surgical margin
Patient Follow-Up
The mean follow-up period from the time of surgery was 28.9 ± 19.4 months. During the follow-up period, 4 patients with a close surgical margin after TORS exhibited locoregional recurrence on surveillance imaging, and 2 patients were confirmed to have locoregional recurrence using biopsy (On-line Figure). Among them, the preoperative MR imaging score was 5 for 2 patients, 4 for 1 patient, and 3 for 1 patient. The other clinico-histopathologic findings of these patients are summarized in On-line Table 3.
DISCUSSION
In this study, we adopted the MR imaging–based scoring system for assessing the involvement of the pharyngeal constrictor muscle in HPV-positive tonsillar SCC and evaluated the predictive value of the MR imaging score for the surgical margin status after TORS. Patients with higher MR imaging scores showed a trend toward positive histologic pharyngeal constrictor muscle invasion by the tumor. An MR imaging score of ≥4 (obscured pharyngeal constrictor muscle by the tumor or parapharyngeal tumor extension) was the single most significant predictive factor of an insecure surgical margin after TORS with an OR of 6.59. Patients with a higher MR imaging score tended to exhibit locoregional recurrence during follow-up, despite the low preoperative cT category of the tumor.
Assessing the anatomic landmarks of the superior pharyngeal constrictor muscle and the parapharyngeal fat is important in the preoperative evaluation of HPV-positive tonsillar SCC before TORS. Tonsillar SCC invading the pharyngeal constrictor muscle on a surgical field is known to increase a risk of locoregional recurrence,26 and tumor invasion of the parapharyngeal fat is likely to leave an insecure surgical margin.5 These structures can be directly visualized on MR imaging, which is an excellent imaging tool for the head and neck with superb soft-tissue contrast and high spatial resolution. In this regard, we hypothesized that preoperative MR imaging could predict the surgical margin status by scoring the degree of constrictor and parapharyngeal space invasion by the initial tumor. This hypothesis was supported by our results, which indicated that the 5-scale MR imaging scoring system could effectively predict insecure surgical margins after TORS.
Our results identified 14 patients who were diagnosed with early cT2 or cT1 cancers but had MR imaging scores of ≥4 (On-line Table 1). These 14 patients underwent TORS as a first-line surgical treatment, but 9 had insecure surgical margins necessitating adjuvant therapy according to the NCCN guidelines. On the basis of this observation, it can be inferred that if we can predict insecure margins before TORS, particularly in patients with early-stage cancer (ie, cT1 or cT2), we can preselect the patients who can receive radiation or chemoradiation instead of surgery as a first-line treatment, despite the low clinical T-stage. Therefore, the preoperative MR imaging scoring system may have a significant clinical impact on treatment selection for patients with HPV-positive tonsillar SCC by predicting the surgical margin.
Our study results have important implications for patients who are candidates for TORS. First, MR imaging scores of 4 and 5 not only were related to the histologic pharyngeal muscle invasion by the tumor but also indicated a high probability of obtaining a positive or close margin. It is known that the invasion of the superior constrictor muscle itself should not lead to a positive margin if the surgery is performed correctly and the muscle is excised as the deep margin. However, our findings suggest that tonsillar SCC seemingly invading the constrictor muscle on MR imaging may include microscopic parapharyngeal fat invasion, and the preoperative work-up cannot guarantee tumor-free parapharyngeal fat.
Second, the selection of candidates for TORS as a first-line treatment has predominantly been based on clinical T-staging to date.2,5,27⇓⇓-30 However, because the status of the constrictor muscle or parapharyngeal fat in HPV-positive tonsillar SCC is not currently applied in the AJCC staging21 or NCCN guidelines,22 there is risk of selecting improper patients for surgery who are predicted to have an insecure margin. We hope that our study result can be a motive for future prospective clinical trials to verify the interrelation between the MR imaging score and clinical staging.
Third, during the follow-up period, 4 patients with early cancer (On-line Table 3) had locoregional recurrence. Despite the low clinical and pathologic staging, the initial MR imaging score for the pharyngeal constrictor of these patients was ≥3, and the final surgical margin was close. We speculate that the preoperative MR imaging score could be associated with locoregional recurrence. However, the number of patients with locoregional recurrence was small, and the follow-up duration was rather short. Future prospective studies that can determine the statistical significance of the relationship between disease-free survival and the MR imaging score are warranted.
Our study had some limitations. First, most of the study subjects were retrospectively analyzed and thus inherently had low pre-TORS clinical and pathologic T-categories of c/pT1 and c/pT2. In addition, the treatment-related factors such as the presence or absence of the types of adjuvant treatment were variable among the patients. However, despite the heterogeneity of the clinical data, we performed the univariable and multivariable logistic regression analyses with Firth correction to overcome this limitation, and they revealed that the MR imaging score for pharyngeal constrictor muscle invasion was a significant predictor of a surgical margin, even after the consideration of the cT and pT categories. Second, the sample size was small, and the mean follow-up period of 28.9 months was too short to firmly establish the role of MR imaging scores in predicting locoregional recurrence. Future prospective studies are warranted on a larger scale, such as a multicenter study, which can determine the relationship between the preoperative MR imaging score and clinical outcome. Third, although parapharyngeal tumor extension is a relative contraindication for TORS,5 we included 6 patients who had MR imaging scores of 5 but received TORS as a first-line treatment. However, the tumor protrusion on MR imaging was not substantial in these patients; thus, we believed that parapharyngeal tumor excision could be performed as reported in a previous article.31
Fourth, there were false-negative and false-positive cases of histologic pharyngeal constrictor muscle invasion when determined by the MR imaging scores of 4 and 5. There was also 1 patient with an MR imaging score of 5, but with a negative surgical margin. We assumed that the reason for the false-negative cases was the microscopic tumor invasion, which could not be detected on MR imaging under its current resolution. In addition, the false-positive cases were probably due to the pushing margin, which means the tumor compressed and pushed the constrictor muscle toward the parapharyngeal fat but preserved the lateral fascia of the superior pharyngeal constrictor muscle near the pterygomandibular raphe. Future advancement in the MR imaging resolution may assist in reducing the false-negative and false-positive cases. Lastly, TORS was performed by 2 surgeons who might have had varied surgical techniques. However, both were highly experienced and equally skilled surgeons. Therefore, we can ensure that the difference in the surgical technique between the 2 surgeons had little influence on the result of the surgical margin.
CONCLUSIONS
The MR imaging–based scoring system is an effective tool for assessing pharyngeal constrictor muscle involvement in HPV-positive tonsillar SCC. An MR imaging score of ≥4 was the single most significant predictive factor of an insecure surgical margin after TORS, independent of the clinical and pathologic staging. Therefore, the preoperative MR imaging scoring system for the pharyngeal constrictor muscle is a promising predictor of the final surgical margin, thereby assisting in the appropriate selection of TORS treatment of HPV-positive tonsillar SCC, even in early T2 tumors.
Acknowledgments
We thank the Medical Research Collaborating Center at Seoul National University Bundang Hospital for assisting with statistical analyses and Editage (www.editage.co.kr) for English language editing.
Footnotes
Yong Ju Kim and Woo-Jin Jeong contributed equally to this article.
This work was supported by the National Research Foundation of Korea grant funded by the Korea government (No. 2019R1F1A1063771) and grant No. 09-2019-003 from the Seoul National University Bundang Hospital Research Fund.
Paper previously presented at: American Society of Head and Neck Radiology 53rd Annual Meeting, where it won the first-place scientific exhibit award, October 2-6, 2019; Phoenix, Arizona.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received January 28, 2020.
- Accepted after revision July 29, 2020.
- © 2020 by American Journal of Neuroradiology