Abstract
SUMMARY: We report 2 children (patients 1 and 2) with Kearns-Sayre syndrome and 1 (patient 3) with Leigh syndrome, who underwent serial diffusion-weighted MR imaging (DWI) studies for 2.8 (patient 1), 4.2 (patient 2), and 1.0 years (patient 3). The DWI revealed the persistent hyperintense signals in the pontine and mesencephalic tegmenta. The apparent diffusion coefficient in the affected regions remained constantly low, suggesting that cytotoxic edema and spongiform degenerations may compose these brain stem lesions.
Neuroradiologic findings of Kearns-Sayre (KSS) and Leigh syndrome (LS) include the bilaterally symmetric bright signals in the basal ganglia and brain stem on T2-weighted or fluid-attenuated inversion recovery (FLAIR) images1; however, the characteristic features on diffusion-weighted MR imaging (DWI) studies and the long-term outcomes remain to be determined. We report 2 children (patients 1 and 2) with KSS and 1 (patient 3) with LS, who underwent the serial DWI studies for 2.8 (patient 1), 4.2 (patient 2), and 1.0 years (patient 3).
Case Reports
Patient 1
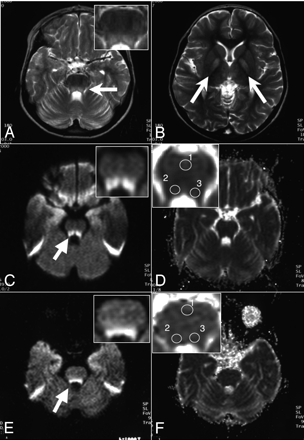
An 11-year-old girl presented with progressive neurologic deterioration, heart block, and multiple dysfunctions in the endocrine systems, including diabetes mellitus and adrenal insufficiency. The diagnosis of KSS was made by the typical clinical presentation, the presence of ragged-red fibers on the muscle specimen, and the large deletions in the mitochondrial DNA (mtDNA). The MR imaging studies with 1.5T Magnetom Vision or Symphony scanners (Siemens, Erlangen, Germany) were performed every 6 months between 6.0 and 8.8 years of age until the cardiac pacemaker was implanted. The apparent diffusion coefficient (ADC) map images were developed from DWI with b factors of 0, 500, and 1000 seconds/mm2. The T2-weighted images at 6 years of age showed symmetric hyperintense lesions in the pontine tegmenta and bilateral globi pallidi (Figs 1A, -B). DWI at this age showed hyperintense signals in the affected area (Fig 1C), whereas the ADC values in these areas (63.3 × 10−5 and 64.8 × 10−5 mm2/s) were lower than those in the reference area (72.3 × 10−5 mm2/s, Table and Fig 1D). The following DWI studies illustrated that the diffusion abnormalities remained unchanged for 1.8 years (Fig 1E). The ADC values in the affected areas (81.3 × 10−5 and 79.4 × 10−5 mm2/s) were lower than those of the reference area (83.6 × 10−5 mm2/s, Table and Fig 1F). The serial DWI of the patient also demonstrated that the basal ganglia had higher signal intensities than the surrounding cerebral white matter, whereas the ADC values within the basal ganglia remained lower than those in the surrounding parenchyma (data not shown).
Selected T2-weighted images, DWI, and ADC maps of patient 1. The axial views of the T2-weighted image (TR/TE, 3203/96.0 ms) of patient 1 show the symmetric hyperintense lesions in the pontine tegmenta (A) and the bilateral basal ganglia (B) at 6 years of age. DWI (b = 1000 s/mm2) displays the hyperintense lesions of the pontine tegmenta at 6 years (C) and 8 years of age (E). The ADC map at 6 years (D) and 8 years of age (F) was obtained from the DWI (C and E). In panels A and C–F, the pontes are shown with higher magnification (insets). Arrows indicate the hyperintense lesions on T2-weighted images and DWI.
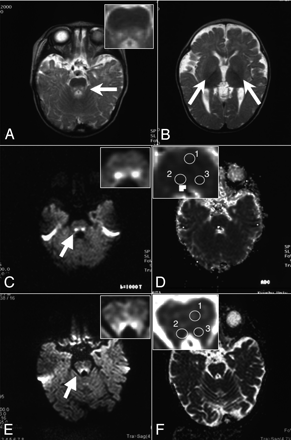
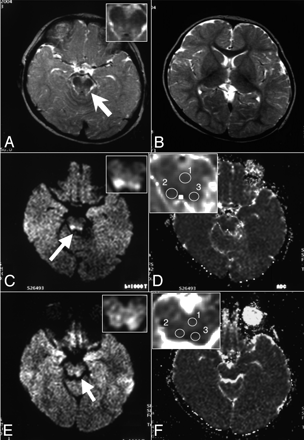
Apparent diffusion coefficient (ADC) values of the hyperintense lesions on diffusion-weighted MR imaging (DWI)
Patient 2
A 6-year-old boy had severe asphyxia and anemia (hemoglobin, 2.9 g/dL) at birth. On the basis of the persistent lactic acidosis, hypoplastic bone marrow, and the large single deletion in the mtDNA of the peripheral lymphocytes, he was diagnosed as having Pearson syndrome (PS). He began to present with the clinical symptoms resembling those of KSS, as commonly observed for patients with PS. In this patient, the hyperintense lesions on T2-weighted images were clearly demonstrated in the pontine and mesencephalic tegmenta at 9 months of age (Fig 2A) but not in the basal ganglia (Fig 2B). DWI also revealed hyperintense signals in the affected regions (Fig 2C), which were still present at 4.0 years of age (Fig 2E). The ADC values of these lesions (88.0 × 10−5 and 87.2 ×10−5 mm2/s) were not lower than those in the unaffected region (87.8 × 10−5 mm2/s) at 9 months (Table and Fig 2D). Nevertheless, the ADC values in the affected regions were reduced to 71.4 × 10−5 and 73.0 × 10−5 mm2/s, which were lower than those in the reference area (77.4 × 10−5 mm2/s) at 4 years of age (Table and Fig 2F). The DWI studies performed between 9 months and 4 years of age demonstrated the constantly low ADC values within the brain stem lesions (data not shown).
Persistent diffusion abnormalities in the brain stem of patient 2. The axial views of the T2-weighted images (TR/TE, 2805/90.0 ms) of patient 2 at 9 months of age show hyperintense lesions in the pons (A) but not in the basal ganglia (B). In panels C–F, the DWI (C, and E) and ADC maps (D and F) at 9 months (C and D) and 4 years of age (E and F) are shown. The pontes are shown in insets of panels A and C–F with higher magnifications. Arrows indicate the abnormal signals on the T2-weighted images and DWIs.
Patient 3
A 25-month-old boy was diagnosed as having LS on the basis of the clinical signs of spastic paraplegia, mental retardation, lactic acidosis, and abnormal neuroradiologic findings on T2-weighted images (Fig 3A, -B). No cardiomyopathy, ophthalmoplegia, retinopathy, hearing loss, or endocrinologic dysfunctions had been observed so far. The hyperintense lesions on T2-weighted images were apparent in the mesencephalic tegmenta (Fig 3A) but not in the basal ganglia (Fig 3B). The affected regions were demonstrated as hyperintense lesions on the DWI at 13 and 25 months of age (Fig 3C, -E). The ADC values of these regions were 71.7 × 10−5 and 85.4 × 10−5 mm2/s at 13 months and 80.3 × 10−5 and 81.1 × 10−5 mm2/s at 2 years of age, indicating that they remained lower than those of the reference areas (94.4 × 10−5 and 88.2 × 10−5 mm2/s) at the respective age (Table and Fig 3D, -F). The DWI performed at 19 months of age also revealed brain stem lesions similar to those at 13 and 25 months of age (data not shown).
Persistent diffusion abnormalities in the brain stem of patient 3. The axial views of the T2-weighted images (TR/TE, 3156/90.0 ms) of patient 3 at 13 months of age demonstrate the symmetric hyperintense lesions in the mesencephalic tegmenta (A) but not in the basal ganglia (B). In panels C–F, the DWI (C and E) and ADC (D and F) maps at 13 months (C and D) and 2 years of age (E and F) are shown. The pontes are shown in insets of panels A and C–F with higher magnifications. Arrows indicate the abnormal signals on the T2-weighted images and DWIs.
Discussion
KSS is characterized by progressive external ophthalmoplegia, the ragged-red appearance of muscle fibers, and the presence of mtDNA with large deletions in the affected tissues.2 Patients with progressive external ophthalmoplegia or KSS with neurologic symptoms had similar findings on T2-weighted images, such as the symmetric lesions lesions at the brain stem and basal ganglia.3 LS is also a progressive neurodegenerative disorder of infancy or early childhood.4 It is characterized by the heterogeneous combination of developmental delay, ataxia, hypotonia, optic atrophy, and lactic acidosis. Neuroimaging studies for patients with LS have disclosed the focal bilaterally symmetric subacute necrosis of the basal ganglia, thalamus, or brain stem regions.3
DWI is an evolving technique that enhances the restricted mobility of water molecules in the brain, and it has been fully appreciated for its capability of detecting the acute ischemic lesions in patients with stroke. The underlying mechanisms of the restricted diffusion after the acute ischemic insults are considered relevant to the depletion of the intracellular adenosine triphosphate because of the decreased cerebral blood flow and the subsequent flux of water into the intracellular space.5 By contrast, the pathophysiology of the diffusion abnormalities associated with the degenerative conditions such as mitochondrial diseases remains to be clarified. The brain stem is known for a tissue highly vulnerable to intracellular energy deprivation. This is evidenced by the fact that brain stem lesions have been documented in patients with acute energy deprivation syndromes, such as Wernicke encephalopathy and LS.6 The neuroradiologic features in the brain stem lesions of Wernicke encephalopathy have been reported to resemble closely those of LS, in which DWI revealed the symmetric midbrain signal-intensity hyperintensities more distinctly than did conventional T2-weighted or FLAIR images.6 Intriguingly, the ADC map showed reduced diffusivity, suggesting the presence of cytotoxic edema within the affected regions.7
Pathologic features in the postmortem brains of patients with KSS and LS include spongiform degenerations, necrosis, and gliosis.8 Such degenerative changes are also found in the brains of patients with Wilson and Creutzfeld-Jacob diseases. Notably, the affected regions in the brains of these patients also exhibited the bright DWI signals with low ADC values.9,10 Vacuolization involving the neuritic processes, the underlying pathologic abnormality in spongiform degeneration, might be responsible for the bright signals on T2-weighted images and DWI in our patients. Vacuolization increases the water flux into the cells and concomitantly decreases the extracellular space, which subsequently reduces the diffusivity of water molecules.10,11 Taken together, the diffusion-weighted findings in our patients may reflect the presence of neurons with persistent cytotoxic edema and spongiform degenerations, which would result in necrosis or gliosis in more advanced stages. In addition, the present radiologic findings of the restricted diffusion are distinct from those of vasogenic edema syndromes, such as mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes, in which the increased ADC values are usually demonstrated from their early stages.12
In conclusion, we found that DWI demonstrated the symmetric hyperintense lesions in the pontine and mesencephalic tegmenta of 3 children with KSS, PS, or LS. The hyperintense lesions on DWI persisted for longer than 1 year, whereas the ADC values of the affected areas remained lower than those of the surrounding areas during the observation periods. Spongiform degeneration or reversible cytotoxic edema may contribute to the formation of the common lesions observed in the brain stem of these mitochondrial diseases.
Acknowledgments
We thank Yasutoshi Koga at the Department of Pediatrics, Kurume University School of Medicine, for helpful comments on the clinical diagnosis of patient 3.
Footnotes
This work was supported by Grant-in-Aid for Scientific Research from Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- Received October 11, 2005.
- Accepted after revision October 23, 2005.
- Copyright © American Society of Neuroradiology