Abstract
BACKGROUND AND PURPOSE: Early disturbances in systemic and cerebral hemodynamics are thought to mediate prematurity-related brain injury. However, the extent to which CBF is perturbed by preterm birth is unknown. Our aim was to compare global and regional CBF in preterm infants with and without brain injury on conventional MR imaging using arterial spin-labeling during the third trimester of ex utero life and to examine the relationship between clinical risk factors and CBF.
MATERIALS AND METHODS: We prospectively enrolled preterm infants younger than 32 weeks' gestational age and <1500 g and performed arterial spin-labeling MR imaging studies. Global and regional CBF in the cerebral cortex, thalami, pons, and cerebellum was quantified. Preterm infants were stratified into those with and without structural brain injury. We further categorized preterm infants by brain injury severity: moderate-severe and mild.
RESULTS: We studied 78 preterm infants: 31 without brain injury and 47 with brain injury (29 with mild and 18 with moderate-severe injury). Global CBF showed a borderline significant increase with increasing gestational age at birth (P = .05) and trended lower in preterm infants with brain injury (P = .07). Similarly, regional CBF was significantly lower in the right thalamus and midpons (P < .05) and trended lower in the midtemporal, left thalamus, and anterior vermis regions (P < .1) in preterm infants with brain injury. Regional CBF in preterm infants with moderate-severe brain injury trended lower in the midpons, right cerebellar hemisphere, and dentate nuclei compared with mild brain injury (P < .1). In addition, a significant, lower regional CBF was associated with ventilation, sepsis, and cesarean delivery (P < .05).
CONCLUSIONS: We report early disturbances in global and regional CBF in preterm infants following brain injury. Regional cerebral perfusion alterations were evident in the thalamus and pons, suggesting regional vulnerability of the developing cerebro-cerebellar circuitry.
ABBREVIATIONS:
- ASL
- arterial spin-labeling
- BI
- brain injury
- DLPF
- dorsolateral prefrontal
- GA
- gestational age
- IO
- inferior occipital
- MT
- midtemporal
- PM
- premotor
- PO
- parieto-occipital
- PT
- preterm
- T1b
- blood longitudinal relaxation time
Premature birth is a major public health concern, with an estimated worldwide incidence of about 9.6% of all births.1 Preterm birth is associated with a high prevalence of brain injury (BI) and life-long neurodevelopmental morbidity, manifesting in up to 50% of school-age survivors.2,3 Overall prematurity-related mortality has decreased in recent years; however, adverse neurodevelopment consequences often persist until later in life.4 Only a few structural brain injuries can be recognized in the early postnatal period (<7–10 days) by conventional transcranial sonography or MR imaging.5 However, most cases of prematurity-related BI lack structural changes; this feature highlights the importance of studying early disturbances in systemic and cerebral hemodynamics that may predispose to such brain injuries.6 Risk factors implicated in prematurity-related BI include alteration of cardiovascular autonomic control, immaturity of the cerebral hemodynamic mechanism, disturbed oxygenation, and vascular fragility.7 The onset and extent to which cerebral blood flow is disturbed following preterm birth remain poorly understood, in large part because of the lack of availability of reliable monitoring techniques that can directly and noninvasively measure CBF.
Several prior studies have assessed cerebral brain perfusion in the neonate using various techniques such as cerebral artery Doppler, near-infrared spectroscopy, PET, and xenon-enhanced CT.8⇓⇓–11 These techniques lack anatomic detail, use indirect cerebral perfusion measurements, and/or have a risk of radiation or the need for intravenous contrast.12⇓–14 Conversely, in the present study, we used the arterial spin-labeling (ASL) MR imaging technique, which has recently emerged as a promising noninvasive method for direct quantitative assessment of cerebral perfusion in high-risk neonates, including neonates with perinatal stroke15 or hypoxic-ischemic encephalopathy,16 without radiation risk or the need for intravenous contrast.13,17
While several ASL-MR imaging studies have been reported in full-term neonates,13,18⇓⇓⇓⇓⇓–24 only 4 studies have performed ASL-MR imaging in the preterm (PT) period (ie, third trimester of ex utero life), all of which included small sample sizes of preterm infants with no structural BI at the time of the MR imaging.12,25⇓–27 To date, to our knowledge, no study has examined early CBF in PT infants during the third trimester of ex utero brain development to better ascertain the impact of early-life BI on cerebral perfusion.
The primary objective of our study was to compare global and regional CBF in PT infants with and without BI on conventional MR imaging during the third trimester of ex utero life using ASL-MR imaging. As a secondary objective, we examined the relationship between regional cerebral and cerebellar perfusion measures and the degree of BI. Last, we investigated clinical risk factors associated with preterm birth and their potential influence on cerebral perfusion.
Materials and Methods
Subjects
In the context of a prospective study, we recruited PT infants (<1500 g and gestational age [GA] of 32 weeks or younger) admitted to the Children's National Medical Center in Washington, DC. PT infants with a known or suspected brain malformation, dysmorphic features, or congenital anomalies suggestive of a genetic syndrome, metabolic disorders, chromosomal abnormality, or CNS infection were excluded. PT infants were stratified into those with and without structural BI on conventional MR imaging. We further categorized PT infants by injury severity: 1) mild injury (grade I/II germinal matrix hemorrhage, punctate cerebellar hemorrhage, and/or mild cerebral white matter injury), and 2) moderate-severe injury (grade III germinal matrix hemorrhage, periventricular hemorrhagic infarction, and/or extensive cerebellar hemorrhage). Medical records were reviewed for all subjects. Specific demographic and clinical variables were extracted including birth weight, GA at birth, GA at MR imaging, sex, Apgar score at 1 and 5 minutes; medications such as pressors, diuretics, caffeine, corticosteroids, indomethacin; sedation 48 hours before MR imaging; resuscitation status such as the use of ventilatory support during the hospital stay; intubation in the 48 hours before MR imaging; patent ductus arteriosus ligation; sepsis; and method of delivery. All data were collected in compliance with Health Information Portability and Accountability Act regulations and approved by the institutional review board (Children's National Health System). Informed consent was acquired from all infant guardians.
MR Imaging and Arterial Spin-Labeling Acquisition
PT infants underwent MR imaging on either 1.5T or 3T scanners (Discovery MR 750 and 450, respectively; GE Healthcare, Milwaukee, Wisconsin). PT infants with specific requirements for a temperature-controlled environment (n = 50, 64%) underwent MR imaging studies on a 1.5T scanner using an MR imaging–compatible incubator with 1-channel receiver and transmitter coils. All other PT infants (n = 28, 36%) underwent MR imaging studies on a 3T scanner using 8 receiver-only head coils.
Our acquisition protocol included axial 3D pseudocontinuous arterial spin-labeling with a spiral k-space trajectory, which was performed with the following scan parameters: TE/TR = 11/4300 ms, FOV = 24 cm, matrix size = 512 × 8, section thickness = 3 mm, and scan time = 3:25 minutes. Anatomic T2-weighted images were acquired with either axial T2 periodically rotated overlapping parallel lines with enhanced reconstruction (TE/TR = 101.7/6313 ms, section thickness = 2 mm) and axial single-shot FSE (TE/TR = 160/1320 ms, section thickness = 2 mm) for a 1.5T scanner, or 3D Cube (GE Healthcare) images (TE/TR = 64/2500 ms, section thickness = 1 mm) for a 3T scanner. Images were reviewed by an experienced pediatric neuroradiologist (J.M) for evidence of BI and structural abnormalities.
Neonates were generally scanned without sedation. Sedation was used on a small subset (n = 10) for clinical indications. All PT infants underwent vital signs monitoring throughout the scan.
Data Analysis/ASL Processing
CBF maps were generated using FuncTool software (GE Healthcare) in milliliters/100 g/minute. The blood longitudinal relaxation time (T1b) was corrected to 1.7 seconds.12 We then created a brain mask of the perfusion map, which was coregistered to anatomic T2-weighted images. Global CBF was calculated from the cerebral perfusion map using FSL software (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki).
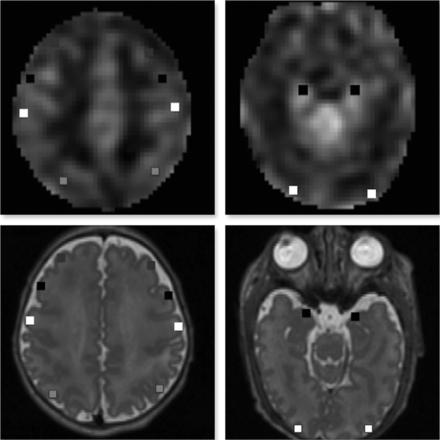
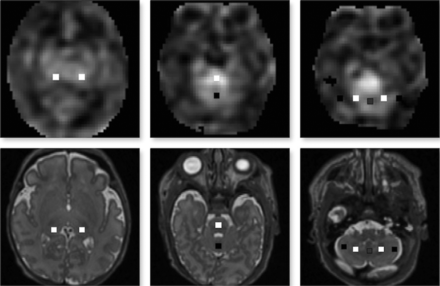
The coregistered CBF map and T2-weighted images were used for anatomic delineation and ROI placement to quantify regional CBF. ROIs were manually placed using ITK-SNAP software (Version 3.4.0; www.itksnap.org) and were calculated as an average value from both the left and right cerebral hemispheres for ROIs that did not show bilateral differences on paired t tests at P < .05. ROIs were drawn as a small square of 2–3 mm in the cerebral cortices in the dorsolateral prefrontal (DLPF), premotor (PM), primary motor, parieto-occipital (PO), midtemporal (MT), and inferior occipital (IO) regions.28 Thalami, midpons, anterior and posterior vermis, dentate nuclei, and cerebellar hemispheres were also included (Figs 1 and 2). The mean volume of ROIs was 23.7–47.2 mm3 (average, 33.06 mm3). The DLPF, PM, primary motor, and PO ROIs were drawn at the level of centrum semiovale and central sulcus. The MT and IO ROIs were drawn at the level of temporal horns of the lateral ventricles. All ROIs were localized by evaluation of the images in 3 orthogonal planes. Intrarater and interrater reliability was assessed via intraclass correlation coefficients on a randomly selected group of 29 PT infants. The mean intraclass correlation coefficients for intrarater reliability measurements ranged from 0.62 to 0.96 (median, 0.82), and for interrater measurements, they ranged from 0.74 to 0.95 (median, 0.90).
ROIs on axial 3D Cube (GE Healthcare) T2-weighted images (lower panel) coregistered with a corresponding CBF map (upper panel). ROI locations include DLPF, PM, primary motor, and PO cortical regions, respectively, (left panel) and MT and IO regions (right panel).
ROIs on axial 3D Cube (GE Healthcare) T2-weighted images (lower panel) coregistered with a corresponding CBF map (upper panel). ROI locations include thalamus regions (left panel), the midpons and anterior vermis (middle panel), and posterior vermis and bilateral dentate and cerebellar hemispheres (right panel).
Statistical Analysis
Descriptive characteristics of PT infants with and without BI were compared using t tests for normally distributed continuous variables, the Wilcoxon-Mann-Whitney test for nonparametric continuous outcomes, and the χ2 test for categoric outcomes. Associations between GA at birth and GA at MR imaging and all CBF measures were evaluated using generalized linear regression. Differences in CBF by ROIs between PT infants with and without BI and by BI severity were evaluated using ANCOVA, adjusted for GA at birth. Additional adjustment for GA at MR imaging (postmenstrual age) was considered; however, GA at MR imaging was not significantly correlated with any ROIs considered. Finally, generalized linear models were constructed for prespecified medical risk factors and all brain regions, controlling for age at birth, to identify medical factors that may be indicative of region-specific decreased CBF. Categoric risk factors identified in <10% of the PT infants were excluded. These included indomethacin and corticosteroid use. Additional analysis controlling for BI status resulted in similar findings. A P value of < .05 was considered a significant cutoff.
Results
Characteristics of the Cohort
We studied 82 PT infants, of whom 4 were excluded due to technical factors (poor quality T2 or ASL). The remaining 78 PT infants had MR imaging at a mean GA of 33.7 ± 2.1 weeks (range, 28.4–37.0 weeks). Of the 78 PT infants, 31 (40%) had structurally normal MR imaging findings (ie, no BI) and 47 (60%) had BIs, of which 18 were moderate-severe and 29 were mild. Descriptive characteristics are summarized in Table 1.
Descriptive characteristics of the cohort (N = 78)
Global CBF Measurement of the Cohort
The adjusted average global CBF showed a borderline significant increase with advancing GA at birth (P = .05), controlling for GA at MR imaging. However, there was no significant difference in global CBF with GA at MR imaging, controlling for GA at birth. The estimated global CBF was 20.3 (95% CI, 18.4–22.3) for PT infants without BI and 18.0 (95% CI, 16.4–19.5) in PT infants with BI, adjusting for GA at birth, indicating that global CBF in PT infants with BI trended lower than in PT infants without BI (P = .07) (Table 2).
Global and regional CBF between PT infants with brain injury and PT infants without injury, adjusted for GA at birtha
Regional CBF Measurement of the Cohort
The adjusted regional CBF was significantly lower in PT infants with BI compared with those without BI in the right thalamus and midpons regions and trended lower in the MT, left thalamus, and anterior vermis regions (Table 2).
Regional CBF in PT infants with moderate-severe injury trended lower in the midpons, right cerebellar hemisphere, and dentate nuclei regions compared with PT infants with mild BI (On-line Table 1).
Clinical Risk Factors Predicting CBF in the Cohort
Significant associations identified with generalized linear models for clinical risk factors and regional CBF are presented in On-line Table 2. Our analysis showed that the presence of sepsis, use of ventilation, intubation in the 48 hours before MR imaging, and cesarean delivery were associated with lower global or regional CBF. However, pressor use was associated with higher CBF. Other risk factors explored were patent ductus arteriosus ligation, diuretic or caffeine use before MR imaging, and sedation 48 hours before MR imaging (On-line Table 3), though no significant associations were noted. Additional analysis controlling for BI status resulted in similar findings.
Influence of 1.5T versus 3T MR Imaging on CBF Measures
We adjusted our analyses for scanner type (1.5T versus 3T) and found no significant differences in global or regional CBF values for our cerebral regions or the vermis or dentate nuclei. Only cerebellar hemispheric CBF was significantly higher at 1.5T compared with 3T (P = .03).
Discussion
In this prospective observational study, we report, for the first time, early disturbances in cerebral perfusion between PT infants with and without structural BI during the third trimester of ex utero development. We demonstrate that global CBF trended slightly lower in PT infants with BI compared with those without. Regional CBF was significantly lower in the right thalamus and midpons and trended lower in the MT, left thalamus, and anterior vermis in PT infants with BI, suggesting regional vulnerability of the developing cerebro-cerebellar circuitry. These results collectively emphasize that perfusion effects in PT infants with BI and specifically moderate-severe injury are directed lower compared with those without BI. Finally, we reveal an association between lower regional CBF and the presence of sepsis, use of ventilation, intubation in the last 48 hours before the MR imaging, and cesarean delivery, after controlling for GA at birth.
Available data on cerebral perfusion using ASL-MR imaging in the neonatal period are limited, particularly available data with MR imaging studies performed during the preterm period (ie, third trimester of ex utero life) or those with prematurity-related brain injury. To date, most studies have been performed either in full-term infants or PT infants at term-equivalent age.13,17⇓⇓⇓⇓⇓⇓–24 Quantitative data on CBF in PT infants during the third trimester of development are scarce. To the best of our knowledge, only 4 studies have performed ASL-MR imaging at preterm age.12,25⇓–27 Varela et al25 scanned <10 PT infants without BI to quantify the ASL parameters and techniques. De Vis et al12 scanned 6 PT infants, looking for cerebral perfusion changes with brain maturation. In another study by the same authors,27 scanning of 18 PT infants was performed to assess hematocrit variability in the T1b and ASL measurements. Ouyang et al26 reported higher global CBF and heterogeneous increases in regional CBF during the third trimester in 17 PT infants. Our data are in keeping with these findings; however, our study is the first to characterize quantitative regional CBF in PT infants with BI.
The mechanisms underlying disturbed CBF in PT infants are undoubtedly complex and likely multifactorial. In part, they may be related to the impact of PT birth on the alteration of cardiovascular autonomic control, which can manifest as variability in heart rate and blood pressure.7 Another potential mechanism may relate to immature cerebral hemodynamics, which increases the risk for cerebral hemorrhage and hypoxic-ischemic injury, especially in extremely premature infants.7,28 Increases in physiologic stress and metabolic demand, either in utero or during the early extrauterine life, may exceed the ability of the increase in cerebral blood flow.7
Our finding of decreased CBF in PT infants with BI corroborates a previous study by Lin et al,29 using near-infrared spectroscopy and diffuse correlation spectroscopy imaging. However, ASL-MR imaging has an advantage over the previous method because it is a direct quantitative technique for assessing cerebral perfusion. Furthermore, it can be combined with other MR imaging techniques (spectroscopy, diffusion, and functional) to give a more comprehensive understanding of the pathophysiologic consequences of early hemodynamic disturbances.14
The decrease in regional CBF in the thalami, midpons, anterior vermis, and dentate nuclei in PT infants with BI suggests a regional vulnerability of the developing cerebro-cerebellar circuitry. This circuitry includes multiple complex closed-loop circuits between the cerebral cortical regions and the cerebellum that control movement, language, and social processing.30 The pivotal structures for this anatomic connection are located within the thalamus and brain stem regions, which represent areas with the highest perfusion during this critical preterm period of brain development. Limperopoulos et al31 reported a significant association between cerebellar injury in PT infants and impairment of regional volumetric growth in the contralateral cerebrum. Arca-Díaz et al32 reported that ADC values in the brain stem and cerebellum can predict the outcomes of neonates with hypoxic-ischemic encephalopathy; this finding further emphasizes the importance of the cerebro-cerebellar connection.
Our analysis of medical risk factors and CBF was primarily exploratory. The potential effects of ventilation or intubation status on CBF are complex.33 Other studies using near-infrared spectroscopy or intravenous 133Xe reported a decrease in CBF in ventilated PT infants,33 which is supported by our data. Most of the ventilated PT infants were on sedative medication at some point during their neonatal intensive care unit stay. It has been shown by van Alfen-van der Velden et al34 that morphine is associated with increased cerebral blood volume, and midazolam, with a decrease in CBF velocity in ventilated PT infants. In our study, we examined the effect of sedative medication used up to 48 hours before MR imaging and found that there were no significant differences in CBF between PT infants who were sedated and those who were not sedated. This finding could be partly related to the small subset of PT infants receiving sedative medications in our cohort (n = 10).
There are several studies that report CBF/cerebral oxygenation differences according to the mode of delivery during the neonatal period.35 Our data further support the notion that cesarean delivery may play a role in cerebral perfusion changes, with decreases in regional CBF noted. The effect of early-onset neonatal sepsis on CBF is complex and depends mainly on the changes in the cerebral autoregulatory mechanism.36 Many of our neonates who had sepsis during their hospital stay have shown a decrease in regional CBF. Our data also demonstrate an increase in regional CBF in PT infants who had vasopressor medication during their first week of life before MR imaging, which further supports the finding that low-dose dopamine can result in a temporary elevation in CBF in PT infants.37
Our study has several strengths: the largest sample size of PT infants scanned at preterm age of the third trimester of development using ASL-MR imaging to date, the inclusion of PT infants with various degrees of structural BI, and the examination of a variety of clinical factors that may influence perfusion. Acknowledging that ASL has been shown to change with GA, we controlled for GA at birth in our analysis.12
Our study also has some limitations, one of which is the use of generic T1b instead of a subject-specific T1 relaxation of the blood, which can be affected by hematocrit level.38 The manual placement of the ROIs is another limitation because it is rater-dependent. However, we achieved good inter- and intrarater reliability measurements. The use of sedation at MR imaging in 10 PT infants may be another confounder to the study; however, comparison analyses between PT infants with and without sedation demonstrated no statistically significant differences in global or regional CBF.
Furthermore, the use of different MR imaging field strengths (1.5T and 3T) is another potential limitation. However, the 2 groups (those studied on 1.5T versus 3T) may not be similar because those PT infants who needed an MR imaging incubator and a temperature-controlled environment due to their medical status were studied on the 1.5T scanner. Our results according to BI status were consistent across both scanners. In other words, the CBF measures in PT infants with BI were consistently lower compared with the PT infants without BI, and this finding is likely not due to scanner differences. Nevertheless, the best way to compare CBF measurement differences between a 1.5T versus 3T scanner would be to compare measurements in the same infants acquired on both scanners on the same day back to back. However, this was not possible in our study, given the acuity of illness of our cohort. Future work on the impact of MR imaging field strength on cerebral and cerebellar CBF measures is warranted.
Finally, the extent to which these early regional CBF disturbances impair subsequent cerebro-cerebellar development and connectivity and functional outcomes in survivors of preterm birth is an intriguing question that deserves further exploration.
Conclusions
We demonstrate that ASL provides a useful noninvasive tool for identifying early cerebral perfusion abnormalities in PT infants with BI. The ability to directly and noninvasively monitor CBF with ASL-MR imaging in the early postnatal period, when PT infants are at greatest risk for BI, is promising and may assist in identifying candidates for future therapeutic targets and measuring treatment effectiveness.
Acknowledgments
We are thankful to all the families that participated in this study. We would like to thank Ms Caitlyn Loucas for her assistance in recruitment (Ms Loucas has no financial disclosure and no conflict of interest to report).
Footnotes
Disclosures: Marni B. Jacobs—RELATED: Grant: National Institutes of Health, Comments: work supported by the Clinical and Translational Science Institute Center Grant awarded to Children's National as part of the Biostatistics, Epidemiology, and Research Design Core*; UNRELATED: Consultancy: PATH, Inova Children's Hospital, Comments: consulting fees paid for scientific merit review (PATH) and statistical analysis (Inova); Grants/Grants Pending: Jain Foundation, ATyr Pharma, Department of Defense, Comments: biostatistician on grants related to neuromuscular disorders*; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Treat-NMD, Comments: Travel paid for scientific review panel for neuromuscular grant submissions. Catherine Limperopoulos—RELATED: Grant: National Institutes of Health RO1HL116585–01, Canadian Institutes of Health Research MOP-81116.* *Money paid to the institution.
This work was supported by the Canadian Institutes of Health Research (MOP-81116) and the National Institutes of Health (R01 HL116585-01).
Paper previously presented, in part, orally at: Annual Meeting of the Radiological Society of North America, November 22–December 2, 2016; Chicago, Illinois. A fellow research award was granted.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received December 23, 2017.
- Accepted after revision March 23, 2018.
- © 2018 by American Journal of Neuroradiology