Abstract
Summary: Using 18F-fluorodeoxyglucose and 11C-diprenorphine positron emission tomography (PET), we investigated alterations in glucose metabolism and opioid receptor binding in a patient with central poststroke pain, which developed after a small pontine hemorrhagic infarction. In comparison with normal databases, reduced 11C-diprenorphine binding was more accentuated than the hypometabolism on the lateral cortical surface contralateral to the symptoms, and a differential abnormal distribution between the tracers was seen in pain-related central structures. These results show that 11C-diprenorphine PET provides unique information for the understanding of central poststroke pain.
Dejérine and Roussy (1) first described central pain in 1906 using the expression thalamic pain based on the findings of thalamic lesions. Central pain is defined by the International Association for the Study of Pain (2) as pain of CNS origin “including thalamic and pseudothalamic pain” as “diffuse, unilateral pain, often burning with allodynia, hypoesthesia, hypalgesia, hyperpathia, dysesthesia, and neurological signs of damage to structures which supply the affected region.” Radiologic studies have shown that lesions that induce central pain may be located at any level along the neuraxis (3) of the CNS, including the brain stem.
Pain-related structures have been identified through changes in regional cerebral blood flow or glucose metabolism, and a few studies have specifically dealt with central poststroke pain (4, 5). Thus far, to the best of our knowledge, only Jones et al (6) have reported changes in 11C-diprenorphine binding in relation to inflammation and pain in a few patients with rheumatoid arthritis.
In this study, alterations in opioid receptor binding, measured with the nonselective ligand 11C-diprenorphine, are reported in a patient with a pontine lesion causing central poststroke pain and compared with changes in glucose metabolism.
Case Report
Clinical Findings
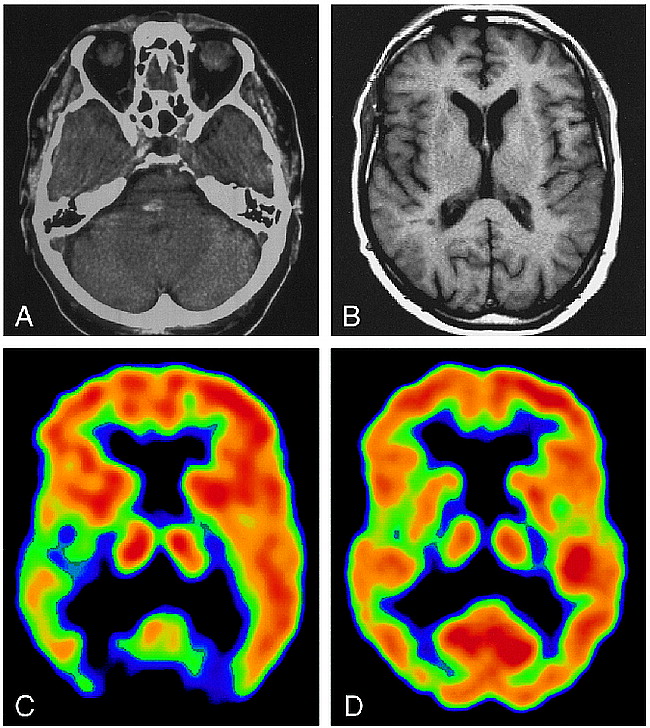
A 77-year-old right-handed man experienced a hemorrhagic brain infarction 14 months before the study. A CT scan (Fig 1A) on the day of the event revealed a hyperdense lesion, approximately 1 cm in diameter, in the right paramedian mid pons region. Motor symptoms dominated initially after the infarction; however, in the following months, a slowly progressive painful dysesthesia of pricking and lacerating character developed on the entire left side of the body. At the time of the study, the pain was continuous, except for cessation after long periods of complete rest. Painful overreactions were provoked by touch or movement, with generalization of pain sensation restricted to the left half of the body and head. The pain was refractory to medical treatment and to physiotherapeutic trials. Medication with opiates (tramadolol hydrochloride, maximum dosage 300 mg/day) resulted only in transient pain relief in the initial period of treatment.
77-year-old patient with central poststroke pain.
A, CT scan on the day of the event shows a hyperdense lesion corresponding to a hemorrhagic vascular infarction.
B, MR image at the time of the PET study, at the same level as C and D.
C, IRF60 image shows a clear difference in opioid receptor binding between the hemispheres, with decreased activity on the right.
D, FDG image shows only slight differences in glucose utilization between left and right sides.
Clinical examination at the time of the study showed slight left-sided distal loss of power, and a mild left-sided spasticity. There was a distinct decrease in sensibility to touch, temperature, and pin-prick on the left side compared with the healthy hemibody. Position sense, vibration, and stereognosis were unimpaired. The patient had a slightly ataxic gait, left-sided dysdiadochokinesia, and poor knee-heel performance. No impairments of mental state (Mini-Mental status examination, 30/30) or speech function were noted, and general physical examination was normal.
At the time of the study, the MR examination (T2*-weighted gradient-echo sequence) revealed a unilateral hypodense lesion of approximately 3 × 6 mm at the location of the infarction. The affected structures were the medial lemniscus and the spinothalamic tract, including trigeminal fibers rostral to the level of contralateral crossing, and the reticular formation. There was no evidence of a thalamic lesion or other cerebral vascular events (Fig 1B).
Electrically evoked somatosensory cerebral potentials showed an increased latency for the left trigeminal, median, peroneal, and femoral nerve, consistent with the radiologic findings. Pain recordings using a heat thermode also indicated a unilateral impairment of the spinothalamic pathways. Nerve conduction velocity, electromyography, electroencephalography, and magnetic evoked potentials were all normal.
Medication directly interfering with the opioid system was discontinued 4 months before positron emission tomography (PET) was performed.
Imaging Findings
The patient and 11 healthy volunteers (mean age, 37; range, 23 to 67 years) underwent a dynamic 11C-diprenorphine study (7) with arterial sampling and metabolite correction (8) after injection of a mean doses of 480 MBq. Additionally, the patient underwent an 18F-fluorodeoxyglucose (FDG)-PET study (370 MBq), with data acquisition between 30 and 60 minutes after injection. FDG-PET data were compared with a normal database consisting of an age-matched control group of 18 healthy volunteers (mean age, 70 years; range, 63 to 82 years). All subjects gave informed written consent. All studies were approved by the Ethics Committee of Medicine of the Technische Universität München and the radiation protection authorities.
Spectral analysis (9) was applied on a voxel-by-voxel basis to extract the opioid receptor availability of 11C-diprenorphine using the impulse response function at 60 minutes (IRF60, [min−1]) (9, 10) (Fig 1C). The regional values of IRF60 and FDG data were assessed by a volume-of-interest (VOI) analysis with exact placement of each VOI, 1.2 cm in diameter, ensured by superimposition onto the individual MR images (11). Additionally, the VOI-derived values were used to calculate asymmetry indexes (AI) between the hemispheres (7) according to the following formula: AI = (left − right) × 100/[(left + right)/2]. An observer-independent automated program was used for the pixelwise statistical analysis (12, 13) of the regional cerebral metabolic rate of glucose. The data sets were normalized to the hemisphere with the higher average value to avoid a possible influence of functional deactivation of the cortical activity by the lesion (4, 5). A region of interest (ROI) analysis was performed on the resulting Z-score maps in the regions equivalent to the VOI. A similar procedure was applied to binding potential images of 11C-diprenorphine, which were generated using the occipital cortex as reference tissue (7, 8, 14). Z-scores presented are average scores in each selected brain region.
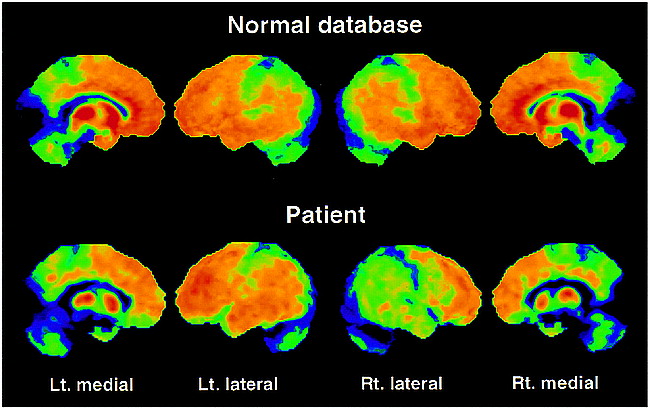
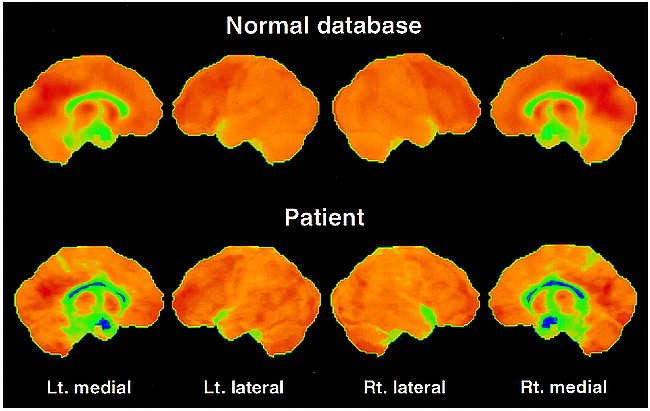
Owing to the high interindividual variance, the absolute quantification of 11C-diprenorphine binding expressed by the IRF60 did not show values outside the normal range (mean value of the lateral cortical hemisphere, 0.1057 − 0.2091 min−1) for any region investigated in the patient (lateral cortical hemispheres: left, 0.1783 min−1; right, 0.1566 min−1). However, the asymmetry indexes calculated from these images revealed a decrease in 11C-diprenorphine binding in the hemisphere contralateral to the clinical symptoms, which was most pronounced in the lateral hemisphere with 3.25 standard deviations (SD) from the control mean (AI-patient: 13.0%; AI-controls: mean, 0.0%, SD, 4.1%) (Figs 1C and 2). This decrease was not matched by an equivalent reduction in FDG uptake (AI-patient: 5.5%, SD, 1.5 from the control mean; AI-control: mean, 1.4%, SD, 2.7%) (Figs 1D and 3). The pixelwise statistical analysis confirmed the difference between the lateral cortical hemispheres, including the frontal, parietal, and temporal cortices except for the primary sensorimotor area with low opioid receptor density (Table). Further, a pronounced relative bilateral (right > left) reduction in opioid receptor binding was shown in pain-related central structures, including the orbitofrontal cortex, the anterior cingulate, and the insula (Table and Fig 2). Within these structures a decrease in glucose metabolism was observed (Table and Fig 3). However, the overall pattern of the impaired FDG uptake and the reduction in 11C-diprenorphine binding was different. Reduction in 11C-diprenorphine binding was more pronounced in anterior parts of the medial surface while hypometabolism was emphasized along the posterior cingulate, an area with lower opioid receptor density (Fig 2).
Diprenorphine binding potential data and FDG data in statistical comparison with control subjects
Surface projections of the 11C-diprenorphine binding potential data of the control mean and the patient viewing the left (Lt.) medial, left lateral, right (Rt.) lateral and right medial surfaces. A marked asymmetry is seen between the left and right lateral surfaces in the patient with less binding on the right cortical surface. An apparent bilateral reduction in binding is identifiable on the medial surfaces of the patient's images in comparison with the control mean. In contrast to the FDG data, reduced 11C-diprenorphine binding is emphasized in the orbitofrontal cortex and in anterior parts of the cingulate cortex (regions with high opioid receptor density).>>
Surface projections of the FDG data of the control mean and the patient from the same views as in figure 2. Upon closer examination, there is a slight asymmetry between the lateral surfaces. More obvious changes in comparison with the control mean are seen on the medial surfaces along the cingulate cortex with a bilateral reduced glucose utilization, especially pronounced in the posterior areas. The hypometabolism at the right upper temporal lobe in the patient is due to a wide sylvian fissure (fig 1B)
Because it was not possible to obtain an age-matched control population for the 11C-diprenorphine study, the possible influence of age on 11C-diprenorphine binding was investigated. Age-related effects on 11C-diprenorphine binding were not found in the literature (14) nor in our database. Gender-specific differences in binding previously described (15) are not relevant in this comparison, since all control subjects were also males.
Discussion
In this study, changes in glucose metabolism and opioid receptor binding in a patient with central poststroke pain were assessed using FDG and the nonselective opioid receptor ligand 11C-diprenorphine. The rare location and limited boundaries of infarction as well as its predominant sensory clinical expression allowed a functional investigation of central poststroke pain in morphologically preserved brain structures.
Although the spectral analysis elegantly offers absolute estimates of receptor availability, the interindividual variance extended over a wide range. This problem is common to all attempts thus far to absolutely quantify receptor-ligand interaction (16). The reference tissue model is a well-validated approach widely used in PET studies with 11C-diprenorphine and other opioid receptor ligands (7, 8, 14). Within a wide, reliable range, the ratio values show a linear relationship to k3/k4 (8), and the images produced are less sensitive to noise artifacts introduced by various parameters necessary for modeling, such as metabolite correction, arterial input function, scale factors, and so on. Therefore, this model was chosen for the statistical comparison to the normal database.
This case study provides the first report of in vivo changes in opioid receptor binding in central pain syndrome after brain infarction. The most likely explanation for the observed decreases in receptor binding in pain-related structures is competition with the exogenous ligand due to tonic enhancement of intrasynaptic endogenous opioid peptide levels, as discussed by Jones et al (6), and/or loss of receptor binding sites (17) caused by increased neurotransmission of the endorphin-mediating analgesia system. Another consequence of the increased levels of endogenous peptides may be a receptor desensitization and opioid tolerance (17), which would also explain the poor response in the patient to potent opioid analgesics. Thus, the data suggest that the alteration of the opioid system affects predominantly structures associated with the medial pain system (cingulate, orbitofrontal cortex, and insula) in concordance with the observations of Jones et al (6) in arthritis patients in the painful state. However, the accentuated reduction in 11C-diprenorphine binding on the lateral cortical surface was not observed in their patients. This can be explained by the peripheral origin of their pain syndrome with no central lesion present. Previous reports of thalamic pain syndromes have described slight metabolic changes in extended areas of the hemisphere contralateral to symptoms (4, 5). The presence of abnormalities in cortical function extending beyond the medial pain system in central pain syndromes is further supported by the findings of this study. Glucose metabolism reflects only total regional synaptic activity in the CNS and does not differentiate between changes in different neurotransmitter systems. In contrast, the imaging of specific neurotransmitter systems, especially the opioid receptor system, may provide unique information for the understanding of pathophysiological mechanisms underlying central pain-related hyperpathia and dysesthesia. Studies in a larger patient population with different lesions along the neuraxis will be necessary to fully elucidate the role of the opioids in the pathophysiology of central pain.
Acknowledgments
We thank V. Cunningham, S. Minoshima, and U. Pietrzyk for generously providing us with their analysis software; M. Rust and his colleagues from the department of anesthesiology for their help; and the technical staff for their assistance. Most appreciated is the careful review of this manuscript by Ngoc Nguyen.
Footnotes
↵1 Supported by the Deutsche Forschungsgesellschaft (SFB 391) and the Norwegian Research Council (project no. 123170/320).
↵2 Address reprint requests to Frode Willoch, MD, Department of Nuclear Medicine, Technische Universität München, Klinikum rechts der Isar, Ismaningerstr. 22, 81675 München, Germany.
References
- Received August 27, 1998.
- Copyright © American Society of Neuroradiology