Abstract
BACKGROUND AND PURPOSE: Although the role of gray-scale sonography for neck nodes is well documented, it plays a limited role in the evaluation of nodal response to treatment. This preliminary limited study sought to determine color duplex sonographic changes in successfully treated metastatic nodes from nasopharyngeal carcinoma.
METHODS: Fourteen patients with nodal metastases from nasopharyngeal carcinoma were studied. A pretreatment sonogram was obtained for all patients. Patients were divided into two groups of seven: in one group, repeat sonograms were obtained 8 weeks after completion of treatment; in the second group, sonograms were obtained 16 weeks after treatment. The features studied included distribution of intranodal vascularity, resistive and pulsatility indexes, and peak systolic velocity. In 11 patients, follow-up sonograms were obtained 1 year after treatment.
RESULTS: The majority (90%) of malignant nodes from nasopharyngeal carcinoma have an increased central and peripheral vascularity, a high resistive index (0.8), and a high pulsatility index (1.8). After radiation therapy to the nodes, a reduction in intranodal vascularity and a statistically significant reduction in the resistive index (0.58 to 0.59) and pulsatility index (0.91 to 0.93) are found. Although a similar reduction in the peak systolic velocity is observed, it is not statistically significant.
CONCLUSION: Our preliminary findings suggest that after radiation therapy for malignant nodes in nasopharyngeal carcinoma, a reduction in intranodal vascularity is found, and the resistive and pulsatility indexes may return to benign parameters as early as 8 weeks after completion of treatment.
Nasopharyngeal carcinoma (NPC) is a common cancer found in Cantonese Chinese, and neck node metastases are common at presentation. Unlike other head and neck cancers, NPC is a radiosensitive tumor, and radiation therapy (RT) is the primary treatment. However, despite adequate treatment, the prevalence of uncontrolled neck disease is 18% (1). The lack of response to initial RT is a poor prognostic factor, associated with a high frequency of recurrent disease.
The role of sonography in the evaluation of neck nodes is well established, particularly when combined with fine-needle aspiration cytology (2). However, sonography has limited use after RT in evaluating nodal response to treatment (3). The introduction of color Doppler sonography has increased the information that can be obtained from a sonographic examination. Newly developed sonographic devices equipped with color flow imaging have greatly increased the ability to detect intranodal vascularity (4) and to provide information about flow and morphologic changes. Findings of recent studies (5–7) suggest that color Doppler sonography can distinguish benign from malignant nodes with a high degree of accuracy (96%) (7). This differentiation is based on the hypothesis that vascular resistance is decreased in vessels of inflamed nodes because of vasodilatation and increased in vessels of malignant nodes because of compression by tumor cells (5). However, the role of color Doppler sonography in evaluating nodal response to treatment is not well documented. We were, therefore, interested in evaluating color duplex sonography in malignant nodes, its change after RT, and its usefulness in determining response to treatment.
Methods
Twenty-two malignant palpable cervical nodes in 14 patients (10 men and four women, aged 31 to 67 years) with NPC were examined prospectively by serial color Doppler sonography. The nodes ranged from 10 to 30 mm in maximum transverse diameter.
All sonograms were obtained with a 10-MHz high-resolution linear transducer (HDI 3000; ATL, Bothell, WA). Sonograms were obtained before the start of RT and repeated at 8 weeks after completion of RT in seven patients and at 16 weeks after RT in seven patients. Nodal response to RT is judged clinically 12 weeks after completion of RT. To determine whether color Doppler sonography can assess nodal response to RT earlier than clinical examination, the patients were randomly divided into two groups of seven: in the first group, sonograms were obtained 4 weeks before clinical evaluation (8 weeks after completion of RT); in the second group, sonograms were obtained 4 weeks after clinical assessment (16 weeks after completion of RT).
The largest node with the most prominent vascularity as shown by color Doppler sonography was selected for the study. This node also underwent a pretreatment sonographically guided fine-needle aspiration cytology before color Doppler sonography to confirm that it was malignant. If the lymphadenopathy was bilateral, one node on each side was chosen. To make sure that the same node was studied on each visit, the location and the relationship of the studied node to adjacent structures were carefully recorded and documented by two sonologists. On each subsequent visit, the sonologists independently reidentified the node. In all cases, absolute consensus between the two sonologists was found. Although the same node was being studied, it is impossible to identify or confirm that the same vessel within the node was examined. However, the vessels with the highest velocity were once again chosen to calculate the resistance index (RI) and pulsatility index (PI).
The blood flow pattern was evaluated with color Doppler sonography. A setting of medium persistence, high sensitivity, low wall filter, and pulse repetition frequency of 700 Hz were used. The settings were standardized for the initial and all subsequent sonograms for each selected node. Qualitatively, the vessel distribution was classified as central, peripheral, or peripheral and central. Assessment of the distribution of intranodal vessels by color Doppler sonography is usually qualitative (7). Although quantification methods are available, they are usually time-consuming and not often used in clinical practice. Doppler waveforms were obtained from eight different regions within the node for the initial sonogram and from as many sites as possible for the subsequent sonograms. The peak systolic velocity (PSV), RI, and PI were obtained at each site with at least three consistent peaks. The three vessels with the highest velocities were chosen to calculate the mean RI and PI in a lymph node. The follow-up sonograms were discontinued when no intranodal vascularity was seen or if the measurements were inconsistent and inaccurate.
None of the patients had any evidence of distant metastases at the time of presentation. All patients underwent either CT or MR imaging for assessment of the primary tumor in the nasopharynx. All patients were treated by RT (60 to 66 Gy over 6 weeks).
After completion of treatment, all patients underwent serial follow-up in the oncology department. This included a detailed clinical examination, nasopharyngoscopy, CT of the nasopharynx, abdominal sonography, and bone scans. In 11 patients, 1 year after completion of the study, repeat sonograms were obtained to evaluate any intranodal vascularity. The remaining three patients were lost to follow-up. Wilcoxon matched pairs signed-rank test was used to assess the nodal response to RT. The response was considered positive when the P value was less than .05
Results
After RT, all patients had a good clinical response, with regression of nodes to an impalpable state.
Vascular Distribution
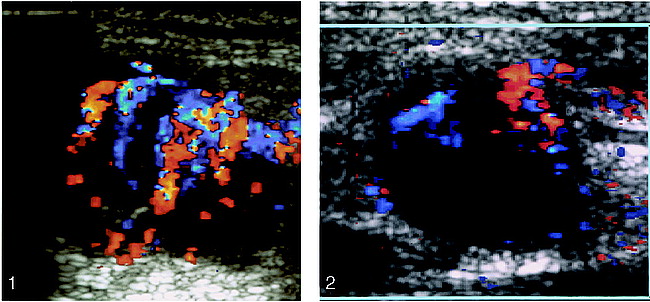
Overall, 20 (90%) of the 22 malignant nodes had increased central and peripheral vascularity (Fig 1), and two (10%) had only increased peripheral vascularity. In the seven patients for whom sonograms were obtained 8 weeks after completion of RT, 12 nodes were detected. Qualitatively, 11 nodes in the pretreatment sonograms had increased central and peripheral vascularity, and one had increased peripheral vascularity.
Enlarged metastatic cervical node from NPC. Color Doppler sonogram shows increased central and peripheral intranodal vascularity. Red indicates flow toward the transducer, blue indicates flow away from the transducer.
fig 2. Same node as in figure 1, 8 weeks after completion of RT. The node remains enlarged, but color Doppler sonogram shows a reduction in the intranodal vascularity. Red indicates flow toward the transducer, blue indicates flow away from the transducer.
After RT, a qualitative reduction in vascularity was found (Fig 2), and vascular signals could be detected in only nine nodes (central vascularity in four, peripheral vascularity in two, and both central and peripheral in the remaining three). In the second group of seven patients who had follow-up sonograms 16 weeks after completion of RT, 10 nodes were detected in the pretreatment sonograms, and, qualitatively, nine nodes had increased central and peripheral vascularity and one had increased peripheral vascularity. Sixteen weeks after completion of RT, the presence of randomly distributed, faint, inconstant vascularity was detected in only two nodes (one central and one peripheral).
Vascular Parameters
Before RT, the mean RI was greater than 0.8, the mean PI was greater than 1.6, and the mean PSV was 21 ± 12 cm/s. After RT, in the group undergoing sonography 8 weeks after completion of RT, the mean RI was 0.58, the mean PI was 0.91, and the mean PSV was 18 ± 10 cm/s. In the group undergoing sonography 16 weeks after completion of RT, in the nodes in which faint intranodal vascularity was detected, the mean RI was 0.59, the mean PI was 0.93, and the mean PSV was 16 ± 9 cm/s. In both groups, only the change in RI and PI was statistically significant. The change in spectral Doppler parameters before and after RT is presented in the Table.
Serial change in the spectral Doppler parameters of nodal vascularity after radiation therapy
One year after completion of the study, all 14 patients remain alive. Of these, six patients are free of disease, with no evidence of local or regional recurrence or distant metastases. Four patients sustained distant metastases but no local or regional recurrence, and one patient had nodal recurrence in the supraclavicular fossa (regional recurrence) but no evidence of local disease or distant metastases.
In 11 patients, follow-up sonograms 1 year after completion of the study showed no consistent intranodal vascularity in the same node as studied previously. In one patient, an impalpable recurrence was detected in the supraclavicular fossa. The distribution of the intranodal vessels and the vascular parameters in this node were similar to RT-naive metastatic nodes from NPC.
Discussion
NPC represents a major cancer risk for Cantonese Chinese not only in China, but wherever they have settled. NPC affects a relatively younger population and, in endemic areas, at least 60% of patients are less than 50 years old (8). The most common complaint of a patient seeking medical attention is the presence of a neck node, and this percentage is even higher in patients younger than 21 years of age (9).
NPC is a radiosensitive tumor, and RT is the primary treatment. Unlike cervical nodes from other head and neck carcinomas, neck node metastases from NPC are generally well controlled by RT. However, recurrent disease is often the problem, because, despite adequate treatment, the prevalence of uncontrolled neck disease is 18% (1). Lack of response to initial RT is a poor prognostic sign, because the recurrence rate of nodes that respond to RT is 13%, compared with 90% if they persist (10).
After completion of the initial treatment, the patient undergoes regular follow-up in the clinic to assess local and regional response and to detect any recurrence. To detect any local recurrence, the patient undergoes nasopharyngoscopy, and, if necessary, CT or MR imaging and a biopsy. Because of post-RT induration, evaluation of nodal disease is a clinical problem, and CT and MR imaging are often indicated for further evaluation. Although the role of gray-scale sonography in the assessment of superficial nodes is well established, its usefulness in the immediate post-RT period is limited (3). Fine-needle aspiration cytology, with a false-negative rate of 39% (11), is also far from dependable in post-RT nodes.
Although color duplex Doppler sonography has been available for some time, it is only recently becoming a routine part of nodal evaluation, and its usefulness in post-RT cases is not yet well documented. Color Doppler sonography is based on the principle that tumor vascularity has certain characteristics that suggest a presumptive diagnosis of malignancy (12–15). The development of high-frequency transducers has improved the ability to detect and examine these vascular signals, particularly from superficial structures such as lymph nodes. In evaluating intranodal vascularity, the parameters assessed include the distribution of intranodal vessels (a qualitative assessment) (7) and intranodal resistance (as reflected by RI and PI).
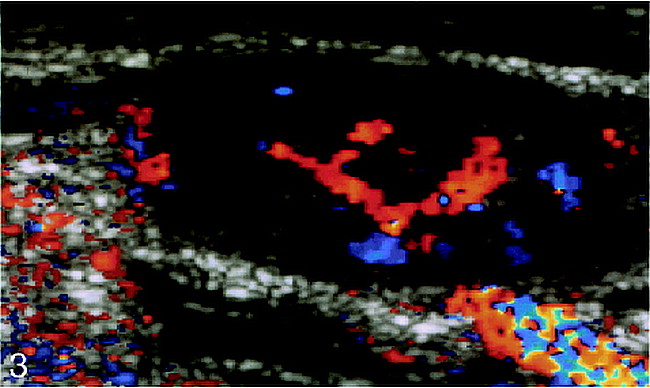
A lymph node has both arterial and venous systems (16). The arterial system consists of a hilar artery, smaller arteries/arterioles in the medulla, and trabeculae and sinus capillaries from arterioles entering the cortex. A few arterioles may reach the capsule via the trabeculae. The veins generally follow the arterial pattern. A normal node has vascularity that appears as a radial and longitudinal configuration with symmetric distribution on color Doppler sonography. In reactive nodal disease, the diffuse nature of the histologic process occurring generally preserves the normal vascular pattern. Enlarged reactive nodes, therefore, have increased hilar perfusion (7) (Fig 3), and because of the supposed vasodilatation (5), a reduction is found in the intranodal resistance, as reflected by a low RI (<0.8) and a low PI (<1.6). In malignant disease, however, the changes in nodal architecture are caused by infiltrating tumor cells that distort and destroy the preexisting nodal architecture, and the increased intranodal resistance (5) resulting from compression by tumor cells is reflected by a high RI (>0.8) and PI (>1.6). The increase in nodal vascularity in metastatic nodes occurs because tumors larger than a few millimeters in diameter stimulate the growth of new vessels by secreting an angiogenesis factor (17, 18). In metastatic nodes, the carcinoma cells are first noted in the marginal and medullary sinuses, progressing to involve both the cortex and medulla. The capsule and perinodal tissues may also be invaded at a later stage (19). This early peripheral involvement of nodes explains the peripheral vascularity detected by color Doppler sonography in the study by Steinkamp et al (7). As the node is progressively involved, increased vascularity is seen in the central and peripheral parts. These changes, therefore, are reflected on color Doppler sonography by a qualitative increase in peripheral vascularity. The PSV in benign and malignant nodes is not significantly different (5).
Enlarged elliptical reactive lymph node. Color Doppler sonogram shows increased central vascularity. Red indicates flow toward the transducer, blue indicates flow away from the transducer
Using an RI threshold of 0.8 and a PI threshold of 1.6, differentiating between reactive and malignant nodes is possible with a sensitivity of 94%, a specificity of 97%, and an accuracy of 96% (7). Another study showed that no hilar flow or a peripheral nodal flow, when combined with a transverse to longitudinal ratio of more than 0.65, had a sensitivity of 92% and a specificity of 100% in depicting metastatic nodes (20). In evaluating nodal vascularity and the effect of RT in malignant nodes from NPC, our study investigated the distribution of vessels within the node and vascular parameters such as RI, PI, and PSV.
Distribution of Intranodal Vessels
Ninety-six percent of malignant nodes have at least one of the four abnormal vascular patterns previously described, including avascular areas, displacement of vessels, increased peripheral vessels, and an aberrant course of central vessels (21, 22). The higher prevalence (90%) of central and peripheral involvement in this study reflects a later stage of nodal involvement, when the entire node is involved by metastases. All the nodes in this study were palpable clinically, reflecting significant nodal involvement. After RT, in patients undergoing sonography 8 weeks after treatment, a qualitative reduction in vessels and vascular signals could be detected in nine nodes. In the group undergoing sonography 16 weeks after completion of RT, faint vascular signals were able to be detected only in two nodes.
Vascular Parameters
Previous studies (5–7) have documented that malignant nodes have a high RI (>0.8) and PI (>1.6). The findings of this study also showed similar results, with an RI greater than 0.8 and a PI greater than 1.6. However, after RT, in the 11 nodes that still had intranodal vascularity, both the RI and the PI had dropped to nonmalignant levels (RI, 0.58/0.59; PI, 0.9/0.93).
Choi et al (5) reported a PSV in malignant nodes of 25 ± 11.7 cm/s and a PSV of 24 ± 16 cm/s in benign nodes. In our study, no significant statistical difference in pre- and post-RT PSV was found. However, PSV is dependent on the angle of examination and, after RT, when the vascularity within the nodes is very faint, these measurements are difficult to obtain and may be inconsistent.
Nodal Response to RT
Nodal response to RT is judged clinically. In this study, all nodes regressed to an impalpable state 12 weeks after completion of RT, which is considered a good clinical response.
Eight weeks after completion of RT, although some nodes remained enlarged (Figs 1 and 2) and had intranodal vascularity, other indicators were found that may suggest a good response. These include a reduction in the number of intranodal vessels (Figs 1 and 2) and a statistical change in the RI and PI to benign criteria. At 16 weeks after RT, the vascular changes were well established.
Despite an initial good response, the presence of a malignant impalpable node in the supraclavicular fossa may be considered a regional recurrence, which may occur in 13% of patients (10). However, a thorough vascular examination of a single node may take about 25 to 30 minutes. In the pretreatment sonograms, although the vessels are clearly seen, consistent and accurate measurements of vascular parameters are time-consuming, since pulsations from the carotid vessels and swallowing may interfere. This problem is further increased in the post-RT phase, during which the vessels are small and often inconstant, and the patient repeatedly swallows because of dryness of mouth after RT. In addition, the equipment required for color Doppler sonography, although readily available in the West, is expensive and may not be available in developing countries. These factors may limit the routine use of color Doppler sonography, particularly in cases in which the response can be confidently assessed by the clinical parameters. In patients in whom the clinical response is borderline or cannot be assessed because of induration after RT, color Doppler sonography is a useful tool in evaluating response, particularly since the other techniques, such as gray-scale sonography, fine-needle aspiration cytology, and CT, are often nondiagnostic (3, 11).
Both MR imaging and positron emission tomography (PET) are useful in evaluating the neck after treatment (23, 24). On MR images, normal posttreatment anatomy is well delineated, revealing good separation of posttreatment fibrosis from normal vascular and muscular landmarks. Posttreatment fibrosis or scarring has been described as being similar or lower in signal intensity than adjacent muscle (25–27). MR imaging also assesses the primary site (nasopharynx), which is not possible with color Doppler sonography. PET is a functional imaging technique that provides information about tissue perfusion and metabolism (28). PET with 18F-fluorodeoxyglucose (FDG) has the added advantage of imaging metabolic changes that appear to be linked to malignancy. Some interest in the evaluation with PET of the therapeutic response of malignant tumors has been expressed. A decrease in FDG uptake has been reported in treated neoplasms after RT (29, 30), whereas elevated FDG activity after RT has been found in persistent or recurrent disease (31). However, the unavailability of PET scans in less developed and developing countries is its main disadvantage.
Limitations of Color Doppler Sonography
Although the findings of this study show that color Doppler sonography is useful in the evaluation of response to RT in large nodes, this technique does not show changes in small impalpable malignant nodes and provides no information about vascularity in nodes that do not respond to treatment. Further studies correlating color Doppler sonography in nonresponding nodes with nodal histology after a radical neck dissection and investigating the role of color Doppler sonography in predicting response in small nodes are necessary.
Another limitation of color Doppler sonography is that it cannot ascertain the primary tumor in the nasopharynx. However, it may have a role in evaluating metastatic nodes in the submental and submandibular areas that are often difficult to image with other techniques because of artifacts from the mandible. Recurrent malignant submental nodes from NPC have been described, and the role of gray-scale sonography has been documented (32). Color Doppler sonography may serve as an adjunct to gray-scale sonography in improving diagnostic accuracy.
Conclusion
This preliminary study suggests that color Doppler sonography may have a role in the follow-up of metastatic nodes from NPC receiving RT. Initial results show that after RT to these nodes, a reduction in the intranodal vascularity and a statistically significant reduction in RI and PI are found. These changes may be seen as early as 8 weeks after completion of treatment.
Footnotes
↵1 Address reprint requests to Dr. Anil T. Ahuja, Department of Diagnostic Radiology, University of Hong Kong, Prince of Wales Hospital. Shatin-N.T., Hong Kong.
References
- Received April 1, 1998.
- Copyright © American Society of Neuroradiology