Abstract
BACKGROUND AND PURPOSE: Respiratory epithelial adenomatoid hamartoma is a benign glandular neoplasm of the sinonasal cavities, which presents in isolation (REAHi) or in the setting of an adjacent inflammatory process such as sinonasal polyps. It is frequently found in the olfactory clefts. CT features of the 2 clinical presentations have not been well defined. We present the CT findings of REAH, focusing on the degree of associated sinusitis and changes in the OCs. We hypothesized that widening of the OCs and associated severity of the sinusitis are diagnostic features of REAH, differentiating it from SNP.
MATERIALS AND METHODS: In this case-control study, we compared patients with REAHi, those with REAH in the setting of SNP (REAHsnp), and those with SNP only (control patients). Patients with REAH were excluded if they had an adjacent inflammatory process other than SNP or if they did not have disease in the OC. We analyzed Harvard sinus CT scores and OC dimensions.
RESULTS: A total of 29 patients with REAH were included: 7 with REAHi and 22 with REAHsnp. A total of 26 control patients were identified. Patients with REAHi had significantly lower Harvard CT scores than did the other groups. The OC width and the ratio of OC to the total nasal distance were significantly larger in both REAH groups compared with those of the control patients. If the OC is 10 mm or more, the sensitivity and specificity for the presence of REAH are 88% and 74%, respectively.
CONCLUSIONS: Both clinical presentations of REAH are associated with OC widening on CT scan. In the setting of polypoid disease, an OC width of > 10 mm should increase suspicion for the presence of REAH.
ABBREVIATIONS:
- OC
- olfactory cleft
- REAH
- respiratory epithelial adenomatoid hamartoma
- SNP
- sinonasal polyposis
- TND
- total nasal distance
Respiratory epithelial adenomatoid hamartoma is a benign neoplasm occurring in the paranasal sinuses, nasal cavity, or nasopharynx. It was first described by Wenig and Heffner1 in 1995 in a case series of 31 patients. Since that time, several case reports and small case series have been published in the pathology and otolaryngology literature, with very limited focus on imaging characteristics.2⇓⇓⇓–6 From a histologic standpoint, REAH is a benign inflammatory polypoid lesion, lined with ciliated respiratory epithelium. Its distinguishing features include an intact basal cell layer and small- to medium-sized glands extending through the stroma, contiguous with the surface epithelium.7⇓⇓–10
Although REAH has been classically recognized as an isolated lesion, further investigation into this clinical entity has revealed that it may also present as an incidental pathologic finding associated with other disease processes in the paranasal sinuses. Most commonly, it occurs with diffuse sinonasal polyposis, but it can also be associated with inverted papilloma or low-grade sinonasal adenocarcinoma.2,4,5,11
REAH in isolation (REAHi) appears as a fleshy pink or tan polypoid, exophytic mass in the nasal cavity and can be unilateral or bilateral. It frequently presents in the olfactory clefts, but it may also be seen emanating from the septum, middle turbinate, or osteomeatal complex. On a CT scan, REAHi can mimic other lesions such as an inverted papilloma, a benign inflammatory polyp, an encephalocele, carcinoma, or an esthesioneuroblastoma. Encephaloceles, carcinomas, and esthesioneuroblastomas are generally associated with skull-base defects or erosions, differentiating them from REAH. However, it is important to note that REAH has been reported to rarely extend intracranially with violation of the cribriform plate(s).6 Fig 1 demonstrates the dramatically different presentations of REAHi and REAHsnp on CT scan.
Coronal CT images demonstrating bilateral OC widening (arrows) in both types of REAH: REAHi (A) and REAHsnp (B). Typical sinonasal panopacification is seen in SNP (B), with an additional finding of OC opacification.
The optimal diagnostic methods and treatment plans for REAH are still under investigation. REAHi seems to be associated with minimal morbidity, but leaving REAHi out of the differential diagnosis may lead to an overly aggressive biopsy procedure, leading to so-called searching for malignant features.2,6 The clinical relevance of REAHsnp remains unclear. To the best of our knowledge, no prospective data have been collected assessing whether REAHsnp has a larger or more clinically significant disease burden than SNP alone. As the clinical implications of this disease process are further investigated, understanding its radiologic features and including it in the differential diagnosis may guide the otolaryngologist in treatment planning.
REAH is a nonenhancing homogeneous mass on CT scan and lacks any defining features. Lima et al12 reported on 15 cases of REAH and found patients to have widened OC on CT scan. However, this study was limited by its small number of patients and its failure to distinguish between REAHi and REAH found in association with other inflammatory processes. We sought to further investigate widening of the OC in patients with REAHi and in those with REAHsnp. We hypothesized that both REAHi and REAHsnp will demonstrate OC widening compared with that in SNP only.
The Harvard CT scoring system14 is a validated tool used to characterize the severity of disease in patients with sinusitis (Table 1). It is frequently used by otolaryngologists as an objective measure to study preoperative and postoperative results from endoscopic sinus surgery.13 If REAH in the setting of SNP signifies a higher disease burden, then patients with REAHsnp may have significantly higher Harvard CT scores compared with scores in control patients. Patients with REAHi are not likely to have any sinus disease because this diagnosis is generally found incidentally on CT or MRI images. Therefore, this group of patients should have the lowest mean Harvard CT scores. We explored both the Harvard CT scoring system and OC measurements as tools to differentiate REAH from nasal polyps.
Harvard CT sinus scoring system14
Materials and Methods
We performed a retrospective chart review to identify patients with a pathologic diagnosis of REAH. Patients with REAH were separated into 2 groups: those with REAHi and those with REAHsnp. A total of 45 patients with REAH were identified, and patients with either isolated REAH or REAH associated with SNP were closely reviewed. Patients with REAH associated with inflammatory processes other than SNP (such as malignancy, inverted papilloma or adenoiditis) were excluded from the REAHsnp group. We identified 11 patients with REAHi and 26 with REAHsnp.
To select control patients, we searched our institutional data base for patients who underwent endoscopic sinus surgery for the treatment of nasal polyps. Using a Microsoft Excel (Redmond, Washington) randomization tool, we then selected 40 patients at random as controls. Patients included in our control group must have had a pathologic review of their surgical specimens demonstrating no evidence of REAH. The first case of REAH at our institution was diagnosed in 2006; therefore, all patients included in our study underwent endoscopic sinus surgery since that time. This protocol avoided having control patients with potentially undiagnosed REAH.
Because we were specifically interested in the findings of the OCs, we excluded patients if they did not have disease in at least one OC on CT scan. After exclusion of such patients, there were 7 patients in the REAHi group, 22 in the REAHsnp group, and 26 control patients.
As we have just mentioned, Harvard sinus CT staging is frequently used by otolaryngologists to characterize sinus disease severity in patients with chronic rhinosinusitis with and without SNP. The scores from each group of patients with REAH were compared with those of the control patients. Available CT scans of all patients were reviewed by a single staff neuroradiologist.
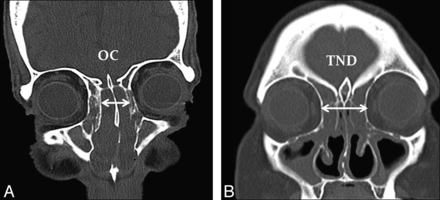
Using the hospital PACS (Impax; Agfa-Gevaert, Mortsel, Belgium), we used direct or reconstructed coronal images of 1- to 3-mm section thickness for measurements (Fig 2). The combined OC width was measured on coronal CT images at its widest point, in the plane traversing through the center of the globes. The TND was then measured between the right and left lamina papyracea in the same plane as the OC measurement. The ratio of OC to TND was calculated. These measurements were then compared among the 3 groups.
Coronal CT images demonstrating the dimensions measured for the OC (A) and the TND (B).
The Fisher exact test was used to analyze categoric data, and the Wilcoxon rank-sum test was used to analyze contiguous data.
Results
A total of 45 patients were diagnosed with REAH from 2006 to 2011 at our institution; 12 had REAHi, and 33 had REAH in the setting of other inflammatory and noninflammatory processes. These processes included SNP (26/33), malignant disease (3/33), inverted papilloma (2/33), hereditary hemorrhagic telangiectasia (1/33), and adenoiditis (1/33). Of all patients with REAH, 78% had disease present in the OCs, mostly bilaterally. Of 12 patients with REAHi, 7 (63%) had disease in the OC (6 bilateral, 1 unilateral). Three patients had lesions along the septum and 2 patients on the medial aspect of the middle turbinate. Of the 26 patients with REAHsnp, 22 (85%) demonstrated opacification of the OC(s) on CT scan (18 bilateral, 4 unilateral). Of the 40 randomly selected control patients, 14 were excluded because no disease was present in the OCs, which left 26 patients in the control group. The mean age of the patients was 68.9 years in the REAHi group, 58.2 years in the REAHsnp group, and 48.0 years in the control group. Patients in the control group were significantly younger than either group of patients with REAH (P < .01). No significant differences were noted in sex ratios, though the patients with REAHi were more likely to be men (Table 2).
Patient population data
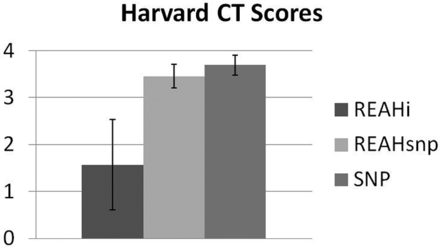
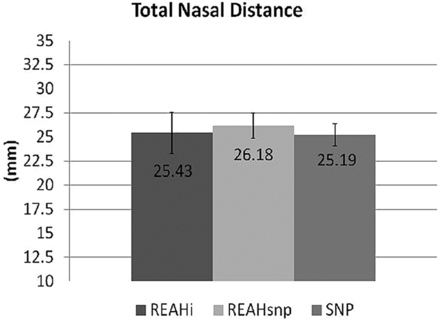
The opacified OCs in both types of REAH were hypoattenuated, without areas of hyperattenuation to suggest trapped fluid with inspissated secretions and/or mineralization. The mean Harvard CT scores were 1.57 in the REAHi group, 3.45 in the REAHsnp group, and 3.69 in the control group. The scores from the REAHi group were significantly lower than the scores of the other 2 groups (P < .001). No difference was noted between patients with REAHsnp and control patients (P = .1889), indicating that both of these groups presented with moderate to severe sinus disease (Fig 3). The OC distance and OC/TND were significantly larger in both groups of patients with REAH compared with control patients (P < .01), and no difference in TND was noted between these groups (Figs 4–5).
Mean Harvard CT scores demonstrating that patients with REAHi have significantly lower scores than the other 2 groups of patients.
Mean width of the OC in 3 groups of patients. *Statistical significance P < .01.
Mean total nasal distance in 3 groups of patients: REAHi, REAHsnp, and SNP.
When comparing all patients with REAH with control patients, we noted that with an OC distance of >10 mm, the sensitivity and specificity for the presence of REAH were 88% and 74%, respectively, yielding a positive and negative predictive value of 72% and 89%, respectively.
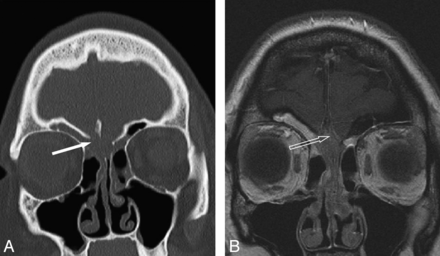
One patient was noted to have bony erosion of the OCs bilaterally. A large mass occupying both olfactory clefts showed T2 hypointensity with restricted diffusion and intracranial extension (Fig 6). A conservative endoscopic biopsy was performed, which revealed the diagnosis of REAH; there were no complications.
Coronal sections of CT sinus demonstrating a bilateral olfactory cleft lesion. A, Erosion of the cribriform plates (solid arrow). B, T1 postcontrast MR imaging differentiating mucus in the ethmoid and frontal sinuses from the centrally located mass with intermediate enhancement. There is an intracranial component (open arrow), with sparing of the dura.
Discussion
As REAH has become more frequently recognized by pathologists, otolaryngologists have begun to investigate its clinical significance. REAH can present in isolation or in association with an adjacent inflammatory process, and both scenarios present distinct diagnostic challenges for the radiologist. REAHsnp is difficult to distinguish from SNP alone, and REAHi can mimic several other polypoid masses.
The significance of incidentally noted REAH on pathologic review remains uncertain, and currently the presence of REAH among diffuse SNP does not change clinical management. However, we were able to demonstrate that a widened olfactory cleft in the setting of diffuse SNP should raise suspicion for the presence of REAH.
We found that disease severity on the basis of Harvard CT scores was no different between patients with REAHsnp and patients with SNP alone. As we excluded patients in our control group who did not have polyps in the OCs, we may have selected patients with worse disease. As expected, patients with REAHi demonstrated significantly lower Harvard CT scores than the other 2 groups. Because these patients are typically asymptomatic, extensive sinusitis noted only on CT scan would be unusual. Although Harvard CT scores have correlated with clinical outcomes, prospective clinical data are needed to further correlate disease severity with the presence of REAH. Of note, sinus CT staging systems (including the Harvard scoring system) do not evaluate for the presence of disease in the olfactory clefts.
Although REAH has been described as a benign lesion and surgical excision has been found to be curative, REAH has been noted within and adjacent to malignant processes. In our series, 3 patients with REAH noted incidentally on pathology studies had disease occurring in association with malignant diseases including squamous cell carcinoma, low-grade intestinal-type adenocarcinoma, and adenoid cystic carcinoma. Jo et al11 reviewed 29 pathologic specimens of patients with nonintestinal low-grade sinonasal adenocarcinoma and found 6 specimens to be associated with REAH. They suggested that REAH may be related to adenocarcinomas or even potentially to a precursor lesion. More likely, as several authors have discussed,2,4,5 REAH is an inflammatory lesion developing in response to the presence of another disease process.
Possibly more relevant to the examining radiologist, an isolated nasal mass presents a much broader differential diagnosis, and REAHi can present with radiographic findings mimicking more worrisome diagnoses such as an esthesioneuroblastoma or an encephalocele. The knowledge of REAH as a potential diagnosis before surgical excision may help to prevent an overly aggressive surgical procedure with greater risk for complications. Such a scenario has been described in prior case reports,2,6 with patients having CSF leak and anosmia. In one of our patients, imaging findings were suspicious for malignant disease, but REAH was also in the differential diagnosis and the patient avoided an extensive skull-base dissection, because a biopsy confirmed REAH. To our knowledge, limited data on MR imaging features of REAH are available in the literature, but studies that have been described are nonspecific, with variable gadolinium enhancement and hypoisointensity on T1.3,14 Unless malignant disease is suspected (suggested by CT findings of skull-base or other bony erosion, or clinical features such as increased vascularity or poorly defined borders), patients with REAH do not typically undergo MR imaging evaluation. This is likely why no significant MR imaging data have been published, to our knowledge.
REAHi arising from locations such as the sinuses or the septum may appear similar to an inflammatory polyp, but the close association with REAH to the OCs makes this entity unique. Olfactory cleft widening and the predilection of REAH to form in this anatomic location is currently the only described feature of REAH distinguishing it from nasal polyps. Hoxworth et al15 showed olfactory cleft opacification as an uncommon finding unless there is an associated inflammatory process or previous surgery, but REAH was not specifically investigated as a cause of OC opacification in this 2008 study. We identified patients with 3 distinct clinical presentations, all with OC opacification. Further characterization of REAH is necessary to distinguish it from other polypoid diseases.
As in the previous study by Lima et al,12 our results also showed olfactory cleft widening on CT scan as a reliable marker of the presence of REAH on histologic examination. Widening of the olfactory cleft may represent bony remodeling from a slow-growing process, similar to what is often seen in a mucocele. It is interesting to note that patients in our control group were significantly younger than both groups of patients with REAH. It may be that the OCs widen with age, or they widen because disease is present for a longer period. Without knowing when the lesions first appeared in our study patients, we are unable to determine if age has a significant effect on the OC distance in these patients.
Our study was limited by its retrospective nature and by potentially selecting patients with SNP with worsened disease severity, as mentioned before. In addition, the small number of patients limits the power of our analysis, but the specific design of our 3 study groups allowed us to compare more homogeneous patient populations. As REAH continues to be recognized more frequently by pathologists, larger series will be amassed, allowing for the clinical significance and radiographic features of REAH to become further elucidated.
In routine endoscopic sinus surgery for SNP, the surgical specimens include tissue removed with so-called cold instruments and a microdebrider. The specimens are generally labeled “right” and “left” sinonasal contents. A biopsy specimen is not traditionally directed toward the OC when the patient has pansinusitis or diffuse SNP. Thus, we cannot definitively conclude that REAH originates in this location. In addition, not all isolated cases of REAH are located in the OC (only 7/11 of our patients with REAHi and 22/26 patients with REAHsnp had disease in the OC). However, most of our patients with REAH were noted to have OC opacification (78%), and the CT scans from those patients characteristically showed significant OC widening. This finding suggests a predilection of REAH to form in this location.
Conclusions
In the setting of an isolated polypoid lesion of the nasal cavity or diffuse SNP, if there is opacification and widening of the OC (>10 mm), then REAH must be suspected. REAHi should be part of the clinician's differential diagnosis to avoid overly aggressive skull-base surgery before biopsy confirmation of a benign lesion. Patients with REAHsnp do not appear to have more severe sinus disease, but prospective data with clinical correlation are needed.
Footnotes
Abstract previously presented at: Annual Meeting of the American Society of Neuroradiology, April 24, 2012; New York, New York.
References
- Received June 6, 2012.
- Accepted after revision July 28, 2012.
- © 2013 by American Journal of Neuroradiology