SUMMARY:
The 2021 World Health Organization Classification of Tumors of the Central Nervous System (CNS5), introduced significant changes, impacting tumors ranging from glial to ependymal neoplasms. Ependymal tumors were previously classified and graded based on histopathology, which had limited clinical and prognostic utility. The updated CNS5 classification now divides ependymomas into 10 subgroups based on anatomic location (supratentorial, posterior fossa, and spinal compartment) and genomic markers. Supratentorial tumors are defined by zinc finger translocation associated (ZFTA) (formerly v-rel avian reticuloendotheliosis viral oncogene [RELA]), or yes-associated protein 1 (YAP1) fusion; posterior fossa tumors are classified into groups A (PFA) and B (PFB), spinal ependymomas are defined by MYCN amplification. Subependymomas are present across all these anatomic compartments. The new classification kept an open category of “not elsewhere classified” or “not otherwise specified” if no pathogenic gene fusion is identified or if the molecular diagnosis is not feasible. Although there is significant overlap in the imaging findings of these tumors, a neuroradiologist needs to be familiar with updated CNS5 classification to understand tumor behavior, for example, the higher tendency for tumor recurrence along the dural flap for ZFTA fusion-positive ependymomas. On imaging, supratentorial ZFTA-fused ependymomas are preferentially located in the cerebral cortex, carrying predominant cystic components. YAP1-MAMLD1-fused ependymomas are intra- or periventricular with prominent multinodular solid components and have significantly better prognosis than ZFTA-fused counterparts. PFA ependymomas are aggressive paramedian masses with frequent calcification, seen in young children, originating from the lateral part of the fourth ventricular roof. PFB ependymomas are usually midline, noncalcified solid-cystic masses seen in adolescents and young adults arising from the fourth ventricular floor. PFA has a poorer prognosis, higher recurrence, and higher metastatic rate than PFB. Myxopapillary spinal ependymomas are now considered grade II due to high recurrence rates. Spinal-MYCN ependymomas are aggressive tumors with frequent leptomeningeal spread, relapse, and poor prognosis. Subependymomas are noninvasive, intraventricular, slow-growing benign tumors with an excellent prognosis. Currently, the molecular classification does not enhance the clinicopathologic understanding of subependymoma and myxopapillary categories. However, given the molecular advancements, this will likely change in the future. This review provides an updated molecular classification of ependymoma, discusses the individual imaging characteristics, and briefly outlines the latest targeted molecular therapies.
ABBREVIATIONS:
- CNS5
- 2021 World Health Organization Classification of Tumors of the Central Nervous System
- EPN
- ependymomas
- EZHIP
- enhancer of zeste homolog inhibitory protein
- MAMLD1
- mastermind-like domain containing 1 gene
- MPE
- myxopapillary ependymoma
- NEC
- not elsewhere classified
- NF2
- neurofibromatosis type 2
- NOS
- not otherwise specified
- OS
- overall survival
- PARP
- poly adenosine diphosphate ribose polymerase
- PFA
- posterior fossa A
- PFB
- posterior fossa B
- PFS
- progression-free survival
- RELA
- v-rel avian reticuloendotheliosis viral oncogene
- ST
- supratentorial
- WHO
- World Health Organization
- YAP1
- yes-associated protein 1
- ZFTA
- zinc finger translocation associated
Ependymal tumors are glial neoplasms that arise from the ependymal remnants of the ventricles, spinal cord, and filum terminale or conus medullaris. They account for 1.8% of all primary brain tumors, with an incident rate of 0.29–0.6 patients per 100,000 population in the United States.1 These can occur in all age groups, with a mean age of 37 years and a slight preponderance in males. Ependymomas are the third most common brain tumor in children, following astrocytoma and medulloblastoma, with over 50% of cases arising in children younger than 5 years of age. Tumor location varies by age, with 90% of pediatric ependymomas occurring intracranially, whereas 65% of adult ependymomas involve the spinal cord. There is increasing evidence that the age of patients influences their grades and survival.2⇓-4
Previously, ependymomas were graded and classified exclusively by histopathology, which has limited clinical utility due to a lack of specificity and reproducibility in predicting outcomes. Ependymomas of the same histologic type often comprise clinically and molecularly distinct subgroups with different clinical behaviors and responses to treatment. Nine molecular subgroups across 3 sites (supratentorial, posterior fossa, and spinal ependymomas) have been identified based on DNA methylation profiling, age at presentation, and prognostic pattern.5 This led to significant changes in the World Health Organization’s (WHO) classifications of ependymal tumors between 2016 and 2021, with the newly updated classification relying primarily on the site of origin and molecular features. The Online Supplemental Data highlight the updates, similarities, and differences between the 2021 and 2016 WHO classifications.6 The histologic categories like “anaplastic” were removed in favor of more objective molecular-based subtypes. No changes were made to myxopapillary ependymoma and subependymoma categories, as the current molecular classification does not provide added clinicopathologic utility for these 2 tumors. The WHO 2021 Classification of Tumors of the Central Nervous System (CNS5), introduces novel genomic markers like zinc finger translocation associated (ZFTA)–fusion (previously v-rel avian reticuloendotheliosis viral oncogene [RELA] fusion), yes-associated protein 1 (YAP1) fusion, posterior fossa A tumors (PFA), posterior fossa B tumors (PFB), and MYCN-amplified spinal ependymoma subgroups, enhancing differentiation based on distinct transcriptional patterns despite similar histology.6⇓-8 Ependymomas lacking a specific molecular alteration are designated “not elsewhere classified [NEC]” and “not otherwise specified [NOS]” when molecular analysis is not possible or failed.6,7,9 Posterior fossa tumors are divided into groups A and B based on DNA methylation pattern, with the former occurring in children and having a poor prognosis. The major change to spinal ependymoma includes the addition of an MYCN-amplified subtype with an aggressive clinical course and high-grade histopathologic features.10 Ependymomas exhibit distinct anatomic preferences. Supratentorial ependymomas are intraparenchymal, posterior fossa tumors are usually intraventricular, and intraspinal tumors are mostly intramedullary.8,9,11 There is significant controversy regarding the clinicopathologic utility of grading ependymal tumors. Despite this, the updated WHO CNS5 advises assigning WHO grade II or grade III to an ependymoma, based on histopathologic features, as part of an integrated brain tumor diagnosis scheme. Fibrillary perivascular pseudorosettes are the histopathologic hallmark of ependymomas.
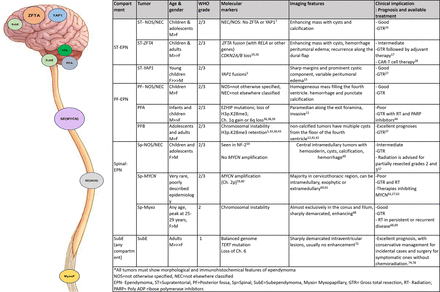
There is significant overlap in the imaging findings of these different subtypes, and distinguishing these tumors based on radiographic findings alone is difficult. Contrast-enhanced MR imaging is the preferred diagnostic technique and forms the cornerstone for diagnosing ependymal neoplasms with gradient-echo sequences depicting hemorrhage and calcification. CT offers a complementary role for better delineation of intratumoral calcification in brain lesions, though it is rarely necessary for spinal tumors. PFA ependymomas are typically paramedian with frequent intratumoral calcifications, contrasting with PFB, which are usually midline with lower calcification rates.12 Multiple recent studies have shown an early trend toward differentiating these lesions based on imaging findings, for example, the higher incidence of intratumoral hemorrhage, cysts, and peritumoral edema in the ZFTA fusion–positive supratentorial ependymoma compared with YAP1 subtype. Unlike supratentorial ependymomas with ZFTA fusion, which are mainly located in the cerebral cortex with significant cystic components, YAP1-MAMLD1-fused ependymomas are found intra- or periventricular with notable multinodular components, regardless of cystic changes. The T2-signal, isointense to the cortex, aligns with the high cellularity of these ependymomas.13 Solid enhancing components in RELA-positive ependymomas can reveal increased vascularity on the perfusion maps, correlating with higher aggressive behavior and chance of recurrence.14 Recent research studies have shown the high performance of deep-learning models for segmentation and molecular subtype prediction in ependymoma, which is expected to grow significantly.15 The molecular subtype also impacts the tumor behavior after surgery, including recurrence rates and chances of leptomeningeal spread, making it essential for the neuroradiologist to be updated with the new classification. There is a strong focus on the molecular classification of tumors during multidisciplinary tumor board discussions. Figure 1 illustrates the typical locations, grade, genetics, imaging features and prognosis of various molecular subtypes of ependymomas, including supratentorial (NOS/NEC, ZFTA, YAP1 fusion), posterior fossa (NOS/NEC, PFA, PFB), spinal (NOS/NEC, MYCN, myxopapillary), and subependymoma. This article reviews the new ependymoma classification and emphasizes the significance of aligning imaging findings with molecular groups.
Diagrammatic representation of the various molecular subtypes of ependymomas in the supratentorial, posterior fossa, and spinal compartments. Table outlining the age-distribution, grade, prognosis, molecular, and imaging features of different molecular subtypes. Anatomic localization forms the basis of the new WHO (2021) classification scheme for ependymomas. Subependymomas are an exception, as they can occur in all 3 anatomic compartments. The supratentorial ependymoma molecular group includes those with fusion genes involving ZFTA (formerly C11orf95) or fusion genes involving YAP1. Methylation profiling divides most posterior fossa ependymomas into 2 main groups, posterior fossa group A (PFA) and group B (PFB), which are also distinguished by levels of H3 p-K27me3. Two molecular groups of spinal ependymomas include myxopapillary morphology or the more aggressive type of spinal ependymoma with MYCN amplification.
Supratentorial Ependymomas
Supratentorial ependymomas (ST-EPNs) present as large tumors localized to the cerebral hemispheres, mostly in children and adolescents and less commonly in adults, often leading to symptoms like headaches, seizures, and focal neurologic impairments. The epidemiology varies according to molecular subtype, with the ZFTA fusion–positive forming most supratentorial ependymomas, especially in children. They have molecular and prognostic features distinct from infratentorial and spinal cord ependymomas. There are 2 main subtypes based on their molecular profiles: ZFTA fusion–positive and YAP1 fusion–positive, and a smaller subset of patients with no definable molecular subtype. The diagnosis of supratentorial ependymoma NEC is used when genetic analysis has not detected a pathogenic fusion gene involving ZFTA (C11orf95) or YAP1. The tumor is categorized as NOS when molecular analysis has been unsuccessful or is not feasible.7 On imaging, these usually present as large intraparenchymal T2 hyperintense lesions, primarily cystic, with peripheral solid nodular enhancement and mass effect (Fig 2). Calcification and cysts are common, whereas necrosis and hemorrhage are rare. Histopathologically, these are grade II or grade III tumors with high cellularity and increased mitotic activity. The differential diagnosis of fusion-negative ST-EPNs includes neoplasms with BCL6 corepressor (BCOR) internal tandem duplication and astroblastomas (Online Supplemental Data). Complete surgical resection is the treatment of choice for long-term survival.16
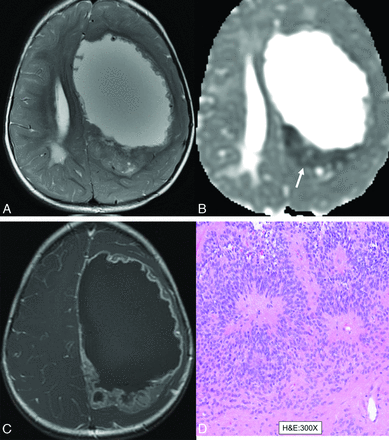
Supratentorial ependymoma, “not otherwise specified” (NOS), in a 1-year-old girl. Solid-cystic heterogeneous T2 signal (A and B) mass in the left frontoparietal lobe with a peripheral rim of nodular contrast enhancement (C) and severe mass effect. Gross total resection was performed with histopathology (D), revealing supratentorial ependymoma, CNS WHO grade III, with high cellularity and increased mitotic activity (up to 14 mitoses per 10 high-powered fields) along with necrosis, supporting an anaplastic designation. The tumor was classified as anaplastic ependymoma at an outside institution with unfeasible molecular testing available. Final integrated diagnosis was supratentorial ependymoma, NOS, given the lack of molecular analysis.
Supratentorial Ependymoma, ZFTA Fusion–Positive.
ST-ZFTAs are circumscribed tumors defined by the ZFTA (formerly C11orf95) fusion gene, replacing the category of RELA fusion in the 2016 WHO classification (Fourth Edition). Fusion of the ZFTA gene is not limited to RELA and can involve other fusion partners like MAML2/3, NCOA1/2, MN1, or CTNNA2 genes, justifying the change.6,17,18 The ZFTA-RELA gene fusion is the primary oncogenic driver, activating NF-κB signaling pathways.19 ST-ZFTA ependymomas are more prevalent in children (66%–84%) than adults (20%–58%) with slight male dominance.5 These tumors are large extraventricular, cortical-based lesions commonly in the frontoparietal lobes originating from lateral and third ventricles.19,20 They can occasionally arise from the thalamus or hypothalamus and rarely at intracranial extra-axial locations.19,21 Clinically, these present with focal neurologic deficits, seizures, and raised intracranial pressure.5
These are large lobulated heterogeneous parenchymal masses with calcification, cystic components, solid-enhancing components, and surrounding edema. Differentiation from other masses can be challenging. Imaging characteristics are similar between the adult and pediatric age groups, with attenuated central calcification and large peripheral cysts (50%).14 CT demonstrates mixed hyperattenuated solid component and hypoattenuated cystic portions with intratumoral calcification. Bony destruction can occasionally be seen in cases with dural-flap recurrence.22 In MR imaging, these are solid-cystic heterogeneous T2 signal masses with FLAIR hyperintense fluid contents and peripheral enhancement of the solid component (Fig 3). Intratumoral hemorrhage, cysts, and peritumoral edema are expected, with the solid component frequently showing low ADC values. MR spectroscopy is nonspecific usually showing decreased NAA, increased choline, with occasionally increased lactate.22 In a recent case series, 9 out of 13 tumors showed circumscribed intraparenchymal cysts with intensely enhancing solid components and notable peritumoral edema. In contrast, 3 tumors were primarily solid with variable enhancement. Restricted diffusion (6 out of 8 patients) and increased vascularity (3 out of 3 patients) on perfusion maps were also observed.17
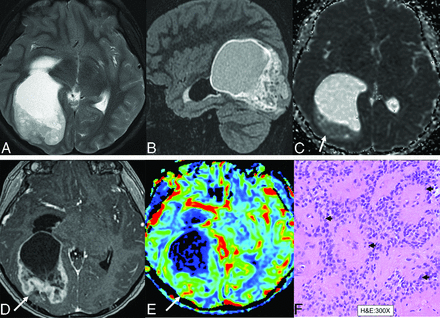
Supratentorial ependymoma, ZFTA fusion–positive, in a 12-year-old girl. Solid-cystic heterogeneous T2 signal (A) mass with T2-FLAIR hyperintense fluid contents (B) with increased ADC values (C, arrow). There is enhancement of the solid component and peripheral walls (D, arrow) with increased vascularity within the solid enhancing component on the CBV maps (E, arrow). Gross-total resection was performed with histopathology revealing fibrillary perivascular pseudorosettes (F, arrows), a hallmark of ependymoma. Molecular testing was performed by a solid tumor fusion analysis, next-generation sequencing (NGS), and DNA methylation profiling. By solid tumor fusion analysis, a ZFTA:RELA fusion was identified. NGS revealed a PIK3CA (p.R88Q) mutation and an approximately 2MB gain on chromosome 11, encompassing ZFTA and RELA, whole arm gain of chromosome 1q, and loss of chromosome 9. By DNA methylation profiling, the tumor matched to methylation class ependymoma, RELA fusion. Overall, the histologic, immunohistochemical, and molecular findings were consistent with supratentorial ependymoma, ZFTA fusion–positive (CNS WHO grade II).
ZFTA-fused ST-EPNs show varying degrees of anaplasia and have been categorized as either CNS WHO grade II or III. These can be distinguished by using molecular profiling techniques, including DNA methylation, and identifying specific chromatic conformations.21,23 Early studies have shown that ZFTA fusion–positive tumors have the poorest prognosis of all ependymomas. However, varying clinical outcomes are noted in some retrospective studies, necessitating validation through a prospective trial.5,20,24,25 Treatment involves complete resection, followed by adjuvant therapy.17 Despite initial management, the prognosis remains grim, with a 10-year progression-free survival (PFS) of around 20% and overall survival (OS) of approximately 50%.5 Homozygous CDKN2A/B deletion in patients with ST-ZFTA indicates a dismal prognosis. As a result, targeting CDKN2A/B inactivation in RELA-ependymomas is suggested as a potential therapeutic strategy.26 Tumor recurrence and leptomeningeal spread are common in ST-ZFTA ependymomas (especially grade III) and usually occur within 2 years of diagnosis. There is also a chance of recurrence along the dural flap in ZFTA(C11orf95)-RELA fusion tumors (Online Supplemental Data).27 While definitive treatment remains elusive, chimeric antigen reception T-cell therapy is one of the novel treatments under exploration for recurrent and metastatic ependymoma.28
Supratentorial Ependymoma, YAP1 Fusion–Positive.
ST-YAP1 fusion ependymomas are less common than ST-ZFTA, comprising around 7% of all ST-ependymomas, with YAP1-MAMLD1 fusion the primary oncogenic driver.5 Ependymoma with YAP1-MAMLD1 fusion is a rare childhood neoplasm, mainly seen in girls younger than 3 years. Despite the large size at initial presentation, the prognosis is significantly better than ST-ZFTA fusion ependymomas, with a 5-year OS of 100%.5,13,25,29 Histopathologically, they exhibit strong EMA immunoreactivity and p65 (RELA) expression and lack L1CAM. They usually present as large supratentorial multinodular cystic tumors with cystic components located in the ventricular or periventricular region and heterogeneous solid enhancing components and variable peritumoral edema, though less pronounced than ZFTA counterpart (Online Supplemental Data).13
Diagnosing supratentorial ependymomas is challenging, with imaging differentials including high-grade pediatric gliomas (except diffuse midline and H3K27-altered types), embryonal tumors, atypical teratoid/rhabdoid tumors, and astroblastoma.8 ZFTA fusion–positive ependymomas have a poor prognosis, whereas YAP1 fusion–positive ependymomas have a better prognosis, despite their larger size at presentation. Tumors in this category have a low recurrence rate and less likelihood of CSF spread and can be observed postresection, without the need of therapy.27 In a study by Espariat et al,30 favorable outcomes with disease-free long-term survival were seen in 29 out of 30 patients.
Posterior Fossa Ependymoma
Posterior fossa EPNs are classified as PFA and PFB subtypes via methylation profiling, and in the absence of this profiling, H3K27me3 levels serve as an alternative marker for differentiation.13,31⇓⇓⇓⇓-36 In 134 adult patients with posterior fossa ependymomas studied, 88.1% were H3K27me3-positive (PFB) and had better survival than the rest, who were H3K27me3-negative (PFA).37 PFA ependymomas are typically paramedian in young children and invade posterior fossa foramina, causing pronounced hydrocephalus, contrasting with PFB, which are midline masses in adolescents and adults, predominantly cystic with noninfiltrative appearance.12,38 Posterior fossa EPNs lacking PFA or PFB are classified as NEC (lacking specific molecular alterations) or NOS (inconclusive or unavailable molecular analysis).
PFA Ependymomas.
PFAs affect infants and young children (median age 3 years), with male predominance. Diagnosing PFA ependymomas requires DNA methylation profiling or loss of H3p.K28me3.36,38,39 PFAs are asymmetric paramedian lesions that mainly originate (75%) from the lateral part of the fourth ventricle roof, extend to adjacent foramen (Luschka, cerebellopontine angle, pontine cistern, and jugular foramen), and invade surrounding structures. These mixed tumors often exhibit intralesional hemorrhage, necrosis, and calcifications, appearing hyperattenuated on CT, with low-signal on T1-weighted images, and heterogeneous signal on T2-weighted images (Fig 4).12,40⇓-42 In a study of 68 patients with posterior fossa EPNs, PFA tumors (56 out of 68; median age: 2 years) were larger (median volume: 57 cm3 versus 29 cm3 in PFB), linked to severe hydrocephalus, and primarily located in the fourth ventricle. PFA tumors frequently invade the foramina of Luschka and often had calcifications (93% versus 40% in PFB) with less tumor enhancement (5% versus 75% for PFB). These tumors also showed fewer cystic components (P = .002) and slightly lower ADC values than PFB tumors (12 out of 68 patients; median age: 20 years).12
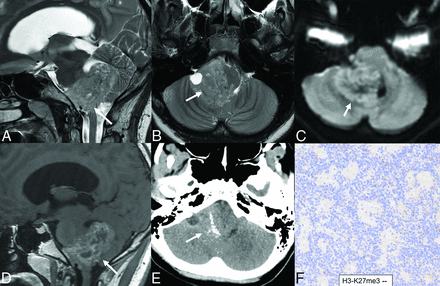
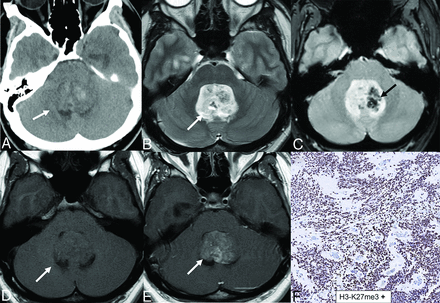
PFA ependymoma in a 17-year-old boy. Predominantly solid, heterogeneous T2 signal (A and B, arrows) mass centered in the right cerebellomedullary cisterns, extending into the fourth ventricle with obstructive hydrocephalus. Lesion shows restricted diffusion (C, arrow) and reveals solid pattern of contrast enhancement (D) with mass effect upon the lower brainstem. Central calcification is seen within the mass on CT (E, arrow). Gross total resection and histopathology revealed posterior fossa ependymoma with high cellularity, elevated mitotic activity (up to 7 mitoses in a single high-power field), microvascular proliferation, and foci of necrosis, corresponding to CNS WHO grade III designation. Widespread loss of nuclear H3-K27me3 expression noted across neoplastic nuclei (E), consistent with the group A molecular group.
About 64% of PFAs show increased mitotic activity and microvascular proliferation; however, these features are inconsistent in predicting the prognosis.38 PFA exhibits CpG hypermethylation, global DNA hypomethylation, variable H3p.K28me3 reduction, and EZH inhibitory protein (EZHIP) overexpression impacting cell regulation. H3K27me3 immunostaining indicates higher invasiveness and correlates with poorer prognosis.38,43 Most PFAs overexpress EZHIP, like the mutant H3K27M, and both inhibit polycomb repressive complex-2, responsible for H3K27me3 deposition. The gain of 1q is an independent poor prognostic marker seen specifically in PFA.35 H3K27me3-negative PF-ependymoma has 5-year PFS and OS rates of 44% and 80%, respectively. Favorable prognostic factors include a Ki-67 index of less than 10%, radiation therapy, and gross total resection.37 PFAs are aggressive tumors that benefit from postresection radiation therapy and respond to poly adenosine diphosphate ribose polymerase (PARP) inhibitors due to elevated EZHIP expression. EZHIP is a key oncogenic driver in PFA that suppresses DNA repair.44 PFA has a poor prognosis, higher recurrence, and metastatic rates than PFB. Survival is significantly linked to surgical extent27, and predicting foraminal invasion improves outcomes.12 In adult PFA cases, 5-year PFS and OS were 54% and 71% for patients over 10 years compared with younger patients.5,45 Therapeutic options for PFA remain limited, but effective medications for H3K27M-mutant diffuse midline gliomas may be promising due to similarities.37
PFB Ependymomas.
PFB ependymomas predominantly affect adult females around the third decade. These are well-circumscribed, spherical, or “ball-like,” and arise midline from the fourth ventricle floor, rarely invading the cerebellum or causing obstructive hydrocephalus.46,47 Imaging shows solid-cystic lesions with necrosis, hemorrhage, and enhancement, giving the common “soap bubble” appearance.46 These small noncalcified tumors have multiple cysts, appear isoattenuated on CT, isointense on T1, and heterogeneous on T2-weighted images with moderate enhancement and higher ADC values (Fig 5).12,41,42 PFB exhibits high chromosomal instability and several cytogenetic abnormalities.5,47 Diagnosis necessitates H3p.K28me3 retention or DNA methylation profiling, with other aberrations including 22q loss, monosomy 6, and trisomy 18 (found in 50%–60% of cases).5,33,36,43 PFBs are seldom invasive or metastatic and have a lower recurrence rate than PFAs. They have excellent prognoses with 5-year PFS and OS rates of 83% and 98%, respectively.37 The prognosis is independent of age at diagnosis but correlates with GTR and Ki-67 < 10%.33,37,45 Common differentials of posterior fossa EPNs include medulloblastoma, characterized by low ADC values due to high cellular attenuation, and pilocytic astrocytoma, which presents as a cystic mass with enhancing eccentric nodule. Subependymomas are well-defined nonenhancing lesions in the older age group and can easily be demarcated from ependymomas.48
Posterior fossa group B (PFB) ependymoma in a 23-year-old man. Solid midline intraventricular mass centered in the fourth ventricle with hyperattenuated hemorrhagic foci on CT (A, arrow). Lesion shows heterogeneous T2 signal (B, arrow) with SWI signal drop-out (C, black arrow) confirming hemorrhagic changes. Lesion appears isointense on T1-weighted image (D, arrow) with moderate contrast enhancement (E, arrow). Tumor resection showed an ependymoma with moderate to high cellularity, moderate proliferative activity (up to 4 mitoses per 10 high power field) and with multiple areas of bland necrosis. There was no evidence of microvascular proliferation, and the histopathologic features were consistent with a CNS WHO grade II ependymoma. The tumor cells showed retained expression of H3K27me3 on immunohistochemical stains (F) consistent with a PFB ependymoma. This finding was confirmed by a whole genome methylation analysis performed. Next-generation sequencing studies were performed that did not disclose the presence of significant mutations and/or fusion.
Spinal Axis Ependymomas
Spinal Ependymoma.
Spinal ependymomas are recognized as distinct genetic entities despite their morphologic similarities to supratentorial and PF-EPNs.5 These tumors arise from neural stem cells in the spinal cord, meninges, and cauda equina, predominantly in the cervicothoracic region, contrasting with lumbar myxopapillary ependymomas. They are mostly low-grade with low recurrence rates following GTR, often rendering adjuvant therapy unnecessary.49 As the primary intramedullary neoplasm in adults and the second most common in children, they account for about 17.6% of adult and 20.6% of pediatric tumors, typically diagnosed between ages 25 and 45.50 Their clinical symptoms, including backache and myelopathy, are nonspecific and resemble other intramedullary tumors.50 Patients with neurofibromatosis type 2 (NF2) often develop ependymoma with frequent chromosome 22q loss and NF2 mutations in spinal ependymomas.51,52 Spinal ependymomas are typically circumscribed soft tumors that occasionally show cysts, calcification, and hemorrhage. These exhibit isomorphic glial cells forming pseudorosettes with low mitotic activity, usually classified as CNS WHO grade II. Grade III cases, though rare, show increased mitotic activity and invasion, requiring differentiation from MYCN-amplified spinal ependymomas and H3K27-altered diffuse midline gliomas. They express GFAP, S-100, vimentin, and EMA, but lack OLIG2 and SOX10 expression (common in astrocytomas and schwannomas). DNA methylation profiling differentiates them from myxopapillary ependymomas, subependymomas, and MYCN-amplified spinal ependymomas.5 The tumor lacks features of myxopapillary ependymoma or subependymoma and lacks MYCN amplification.9,27,53
Spinal ependymomas are hypointense on T1-weighted and hyperintense on T2-weighted images, with solid enhancing components, cysts, hemorrhage, necrosis, and calcification. Around 60% have intramedullary cysts with the “cap sign,” marked by peripheral T2 hypointensity due to hemosiderin deposition.49 Multiple intramedullary ependymomas are known to occur in patients with NF2 (Fig 6).52 Astrocytoma, cavernous malformations, and diffuse midline gliomas are the main differential diagnoses. Spinal astrocytoma, the primary pediatric spinal cord tumor, affects longer cord segments with less distinct borders. Cavernous malformations exhibit multilobulated “popcorn-like” appearance due to the complete hemosiderin rim, usually without perilesional edema.54 Spinal ependymomas are slow-growing tumors with excellent OS after complete tumor excision. Radiation is advised for partially resected grades II and III. Due to limited pediatric studies, pediatric intramedullary ependymoma treatment is based on adult data. They have positive outcomes, with 70%–90% PFS and 90%–100% OS.55 Complete removal and tumor grade are key prognostic factors of long-term tumor-free survival.56
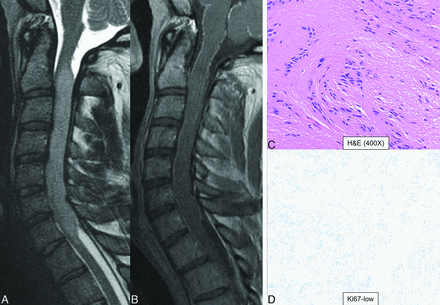
Multifocal spinal ependymoma, NOS, in a 42-year-old woman. Multifocal T2 hyperintense (A) enhancing (B and C) neoplasms throughout the spinal cord in the central region. Neoplasm shows perivascular pseudorosettes on H&E stains (D) with scattered mitotic activity (up to 3 mitoses per 10 high-power fields) and no microvascular proliferation or necrosis, suggesting a low-grade designation. Genomic alterations include gain of chromosomes 5 and 9, and loss of chromosomes 13 and 22. The tumor lacked features of myxopapillary ependymoma or subependymoma, and testing for MYCN amplification was negative. Despite the absence of vestibular schwannoma and any significant family history, the pattern of alterations was consistent with NF-2. Given the absence of MYCN amplification, these tumors are now classified under spinal ependymoma, NOS.
Spinal Ependymoma, MYCN-Amplified.
The newly identified spinal ependymoma (SP)-MYCN molecular subgroup, with heightened MYCN amplification, lacks a comprehensive understanding of its epidemiologic, clinical, and biologic traits. This subgroup shows aggressive behavior with frequent leptomeningeal spread and relapse, leading to a poor prognosis akin to WHO grade III anaplastic ependymomas, despite aggressive treatment.57 The MYCN gene, a part of the MYC family proto-oncogene (chromosome 2p24), regulates cell growth and is found in certain aggressive medulloblastomas and glioblastomas.58,59 SP-MYCN ependymomas are large tumors, mainly in the cervicothoracic cord, spanning multiple vertebral levels. Unlike classic spinal ependymomas with intramedullary location, these usually grow extramedullary and frequently present with leptomeningeal dissemination (Fig 7). These are usually seen in individuals in their 30s, with a slight female dominance.59,60 Rare cases of systemic metastasis have been reported, including metastasis to the humerus and the paraspinal musculature.60 Their OS and PFS rates resemble aggressive tumors like ST-ZFTA and PFA-ependymomas.10 In a study by Ghasemi et al,60 a cohort of 71 SP-MYCN cases showed median PFS and OS of 15.5 and 85 months, respectively. These tumors exhibited high-grade anaplastic characteristics like increased cellularity, cytologic anaplasia, perivascular pseudorosettes, high mitotic activity, necrotic regions, microvascular proliferation, and a lack of H3K27me3 with positive GFAP immunostaining. Various strategies for inhibiting MYCN are under investigation, including HDAC inhibitors, PARP inhibitors, Aurora A-kinase inhibitors, and BET-bromodomain inhibitors, as promising candidates for translation into therapy for MYCN-amplified tumors.27,61
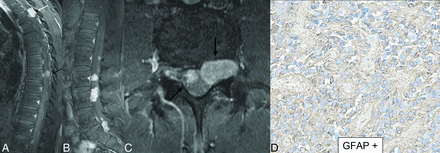
Multifocal spinal ependymoma, MYCN-amplified, in a 29-year-old man. Sagittal (A and B) and axial (C) postcontrast MR images reveal few intramedullary (A, white arrow) and multiple extramedullary (A, black arrows) tumors of varying sizes throughout the thoracic cord and cauda equina nerve roots (A–C). Immunohistochemistry shows many tumor cells expressing GFAP (D) in perivascular radiating processes (pseudorosettes). Tumor revealed high-grade anaplastic histopathologic features, including high mitotic activity, and microvascular proliferation. Chromosomal microarray analysis revealed genomic alterations, including amplification of 2p24.3 (including MYCN), loss of chromosome 17, and gain of chromosomes 4, 7, 8. 9. The pattern of alterations was consistent with spinal ependymoma, with amplification of MYCN.
Myxopapillary Ependymoma.
Myxopapillary ependymomas (MPEs), once classified as grade I tumors, are now considered grade II due to the identification of higher recurrence rates.6,7,9 These slow-growing benign neoplasms originate from the ependymal cells of conus medullaris, cauda equina, and filum terminale and are rarely found in the cervicothoracic and brain. They constitute about 13% of all spinal ependymomas and 83% of the conus medullaris and cauda equina region, typically affecting males around 35 years.9,49,62 The typical symptoms include low backache, leg weakness, and sphincter dysfunction.62,63 While outcomes are generally good, locoregional recurrence and metastatic spread can occur, occasionally with extra-CNS ependymoma metastases.64,65 Macroscopically, they appear as lobulated, sausage-shaped tumors with hemorrhage, calcification, and cystic degeneration. Microscopically, these tumors feature papillary elements surrounding a hyalinized fibrovascular core, forming perivascular pseudorosettes with myxoid material. They also demonstrate positive GFAP, S-100, vimentin, and CD99 staining.66 MR imaging is crucial for preoperative planning and assessing surgical resectability. These well-defined intradural tumors range from small oval tumors displacing cauda equina nerve roots to large sausage-shaped tumors spanning multiple levels. They present as heterogeneous masses with necrosis, calcification, cystic changes, and hemorrhage. They are typically isointense on T1-weighted images, occasionally showing T1 hyperintensity from mucin, calcification, or hemorrhage. These masses are hyperintense on T2 with a distinct low-intensity margin (“cap sign” due to hemorrhage) and variable enhancement. The differentials for small conus medullaris/filum terminale myxopapillary ependymomas include schwannoma and cauda equina neuroendocrine tumors. In contrast, larger tumors causing sacral destruction need to be differentiated from aneurysmal bone cysts, chordomas, and giant cell tumors.67 Myxopapillary ependymomas, typically slow-growing tumors, can disseminate sporadically (14%–43% cases), mainly in the pediatric age group.7 Conus medullaris involvement complicates resection, increasing risk of recurrence and neurologic deficits.62,63 Complete excision may not be feasible due to locally advanced growth and/or cerebrospinal seeding. Surgery with adjuvant therapy is considered in persistent or recurrent disease.67,68 Despite GTR, pediatric MPE often spreads along the neural axis. It mandates regular clinical and radiologic monitoring.68 The 5-year survival rate exceeds 98% after complete excision.7 Recurrence rates and 10-year PFS for MPE varied from institution to institution. In a study of 72 patients with spinal MPE (2011–2021), Zhang et al,65 demonstrated that complete removal with adjuvant radiation improved PFS, with a recurrence rate of 18.8%. Preoperative drop metastasis and sacrococcygeal involvement are associated with a high recurrence rate.
Subependymoma
Subependymomas are noninvasive, intraventricular, slow-growing benign tumors, usually present in middle-aged men with age at presentation ranging from 40–84 years.69,70 Small subependymomas (<2 cm) are often diagnosed incidentally and appear as sharply demarcated nodular lesions bulging into the ventricle, while larger lesions may present with mass effect or CSF obstruction. The most frequent sites are the fourth (60%) and lateral ventricles (30–35%), with occasional occurrences in the third ventricle, spinal cord, cerebrum, cerebellum, bulbar regions, and cerebellopontine angle.71 Spinal subependymomas can rarely cause cord or nerve root compression. These tumors are classified as WHO grade I and no specific molecular markers have been identified at present.6,7,69
Microscopically, they are characterized by clusters of isomorphic nuclei embedded within a gliofibrillary matrix, cystic degeneration, hyalinized vessels without perivascular pseudorosettes, and exhibit low proliferative activity and Ki-67. They show strong GFAP positivity and retain ATRX expression in immunohistochemistry.72 On CT, they present as lobulated, well-defined hypoattenuated or isoattenuated intraventricular masses. On MR imaging, these are well-circumscribed and appear hypo- to isointense on T1-weighted and hyperintense on T2-weighted images. These tumors do not enhance and lack restricted diffusion or peritumoral edema. Heterogeneous T1 and T2 signals may be seen secondary to necrosis, calcification, cystic degeneration, and hemorrhage. Infratentorial tumors display more enhancement, cystic changes, and calcification compared with supratentorial subependymomas. Isolated intraventricular subependymomas are common, while bilateral ventricular cases are rare, with only 4 reported cases.73 Rich microvasculature in certain areas can lead to microhemorrhages, potentially resulting in subarachnoid hemorrhage, acute mass effect, and obstructive hydrocephalus.74 Spinal subependymoma, akin to ependymoma, is a slow-growing benign tumor that needs to be considered in the differential when the tumor is eccentrically located and hard to separate from the spinal cord. MR imaging shows iso- to low signal intensity on T1-weighted and high signal on T2-weighted images, along with poor enhancement, occasional cysts, and calcification (Fig 8).75 Subependymomas have an excellent prognosis, with conservative management for incidental cases and surgery for symptomatic ones without chemoradiation. Recurrences are low (7.9%), with rare subependymal seeding or anaplastic progression cases.72,76
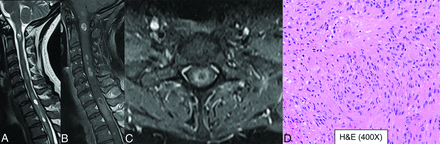
Spinal subependymoma in a 27-year-old man. Long-segment homogeneous T2 hyperintense (A) expansile lesion within the cervical cord with no contrast enhancement (B). The neoplasm shows predominantly subependymoma morphology with clusters of isomorphic nuclei embedded in an attenuated, fine, glial fibrillary background (C) and harbors low proliferative activity with low Ki-67 (labeling index <1%) (D). Lesion did not show significant anaplastic histologic features and did not harbor TERT promoter mutation, MYCN-amplification, or any chromosomal copy number alterations, supporting the diagnosis of subependymoma, CNS WHO grade I.
CONCLUSIONS
The molecular profile of ependymomas offers valuable insights into tumor behavior, clinical course, and prognosis, paving the way for targeted therapies. The recently introduced WHO 2021 classification, which is entirely genomic based, is predicted to evolve into a more accurate and unbiased system, as more data become available. This has become an essential topic in multidisciplinary neuro-oncology forums. Neuroradiologists, who are integral members of these tumor boards, must keep up-to-date with these advancements. Although this field is still emerging, we foresee a significant amount of research soon, focusing on identifying these subtypes through imaging techniques and creating advanced AI models to aid in diagnosis.
Footnotes
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received January 2, 2024.
- Accepted after revision February 8, 2024.
- © 2024 by American Journal of Neuroradiology