Abstract
BACKGROUND AND PURPOSE: WHO grade 3 meningiomas are rare and poorly understood and have a higher propensity for recurrence, metastasis, and worsened clinical outcomes compared with lower-grade meningiomas. The purpose of our study was to prospectively evaluate the molecular profile, PET characteristics, and outcomes of patients with World Health Organization grade 3 meningiomas who were imaged with gallium 68 (68Ga) DOTATATE PET/MR imaging.
MATERIALS AND METHODS: Patients with World Health Organization grade 3 meningiomas enrolled in our prospective observational cohort evaluating the utility of (68Ga) DOTATATE PET/MR imaging in somatostatin receptor positive brain tumors were included. We stratified patients by de novo–versus–secondary-progressive status and evaluated the differences in the PET standard uptake value, molecular profiles, and clinical outcomes.
RESULTS: Patients met the inclusion criteria (secondary-progressive: 7/14; de novo: 7/14). The secondary-progressive cohort had a significantly higher per-patient number of surgeries (4.1 versus 1.6; P = .011) and trended toward a higher number of radiation therapy courses (2.4 versus 1.6; P = .23) and cumulative radiation therapy doses (106Gy versus 68.3Gy; P = .31). The secondary-progressive cohort had a significantly lower progression-free survival compared with the de novo cohort (4.8 versus 37.7 months; P = .004). Secondary-progressive tumors had distinct molecular pathology profiles with higher numbers of mutations (3.5 versus 1.2; P = .024). Secondary-progressive tumors demonstrated higher PET standard uptake values (17.1 versus 12.4; P = .0021).
CONCLUSIONS: Our study confirms prior work illustrating distinct clinical outcomes in secondary-progressive and de novo World Health Organization grade 3 meningiomas. Furthermore, our findings support (68Ga) DOTATATE PET/MR imaging as a useful management strategy in World Health Organization grade 3 meningiomas and provide insight into meningioma biology, as well as clinical management implications.
ABBREVIATIONS:
- GTR
- gross total resection
- PFS
- progression-free survival
- RANO
- Response Assessment in Neuro-Oncology
- RT
- radiation therapy
- SSS
- superior sagittal sinus
- SSTR
- somatostatin-receptor
- SUV
- standard uptake value
- SUVR
- SUV ratio
- WHO
- World Health Organization
Summary Section:
Previous Literature:
WHO grade 3 meningiomas are rare, representing only 2% of all meningiomas, and are associated with high morbidity and mortality. MRI has significant limitations in meningioma evaluation, especially in intermediate- and high-risk tumors. [68Ga]DOTATATE PET has demonstrated high utility in meningioma evaluation and treatment planning. There thus exists a marked unmet need for improved targeted imaging strategies in the management of WHO grade 3 meningiomas. In this study, we evaluate the imaging characteristics, molecular profile, and clinical outcomes of patients with WHO grade 3 meningiomas who were imaged with [68Ga]DOTATATE PET/MRI.
Key Findings:
We found patients with secondary progressive WHO grade 3 meningiomas to have worse progression-free survival (PFS) compared to those with de novo disease, concordant with prior literature. We further found that the secondary progressive cohort demonstrated higher lesion SUV on [68Ga]DOTATATE PET/MRI, as well as increased rate of mutations.
Knowledge Advancements:
This work supports [68Ga]DOTATATE PET/MRI as a management strategy in WHO grade 3 meningiomas and raises the possibility of non-invasively differentiating secondary progressive and de novo meningiomas. Additionally, this work raises important questions regarding meningioma biology such as the role of SSTR2 signaling and mutation rate in higher grade meningiomas.
Meningiomas are the most common primary brain tumor, accounting for approximately 40% of all intracranial lesions.1 The World Health Organization (WHO) classification criteria stratify meningiomas into 3 grades based on the frequency of mitotic figures as evaluated histologically. Most meningiomas are WHO grade 1 and have a favorable prognosis.1 However, a smaller subset are classified as either WHO grade 2 or 3 meningiomas and are associated with greater morbidity and mortality.1
WHO grade 3 meningiomas, which account for <2% of all graded cases, are particularly aggressive with a higher propensity for recurrence, metastases, and worse clinical outcomes compared with lower-grade meningiomas.1,2 While patients with WHO grade 3 meningiomas are typically treated with maximal surgical resection and radiation therapy (RT), prognosis is poor with 5-year recurrence rates of 50%–90% after surgery and 5-year overall survival rates ranging from 20% to 50%.1,3 The 2021 WHO classification defines WHO grade 3 meningiomas both histologically and molecularly. Histologic criteria for grade 3 meningiomas include ≥20 mitotic figures per 10 high-power (400x) fields, well-formed papillary or rhabdoid architecture (typically supported by the presence of PBRM1 and BAP1 mutations, respectively), or frank histologic anaplasia with sarcomatoid, melanomatoid, or carcinomatoid architecture, while molecular criteria for grade 3 meningiomas include the presence of a TERT promoter mutation or homozygous deletion of CDKN2A/B. They can arise de novo or from secondary progression of meningiomas of lower histologic grade, the latter of which are known to have poorer clinical outcomes such as markedly decreased progression-free survival (PFS).2,4
While WHO grade has been used widely to guide clinical decision-making and treatment-planning in meningiomas, histopathologic findings alone have proved suboptimal in predicting clinical and biologic behavior and prognosis of meningiomas. For instance, many cases of WHO grade 1 meningioma will progress with unexpectedly early recurrences, while many WHO grade 2 meningiomas will remain indolent and benign for the entirety of their clinical course.5 WHO grade 3 meningiomas are even less predictable and poorly understood compared with their lower-grade counterparts. Therefore, additional understanding of the diagnosis, management, and prognostic factors surrounding WHO grade 3 meningiomas is needed to optimize treatment-planning and patient outcomes.
The primary neuroimaging approach for the evaluation of meningiomas is contrast-enhanced MR imaging. However, MR imaging has considerable limitations such as in the distinction of recurrent or residual disease from postoperative inflammation and scarring, especially in intermediate- and high-risk meningiomas, further compounded in the context of prior RT.6 More recently, gallium 68 (68Ga) DOTATATE PET has demonstrated utility in meningioma evaluation, including diagnostic confirmation, surgical planning, delineation of radiation therapy target volumes, and posttreatment surveillance.7 By targeting a receptor highly expressed on the surface of meningiomas, somatostatin receptor 2 (SSTR2), (68Ga) DOTATATE PET has contributed to improved molecular imaging-guided management of meningiomas.7,8
The potential role of (68Ga) DOTATATE PET/MR imaging in the characterization of WHO 3 meningiomas has not been previously studied. Here, we evaluate the clinical, pathologic, imaging characteristics, and outcomes of patients with WHO grade 3 meningiomas in our prospective observational cohort of patients with meningiomas undergoing (68Ga) DOTATATE PET/MR imaging and evaluate the differences in PET findings between patients with de novo and those with secondary-progressive WHO grade 3 meningiomas.
MATERIALS AND METHODS
Patient Population
In this institutional review board at Weill Cornell Medicine–approved study, a total of 151 patients with clinically suspected or histologically proved meningiomas underwent (68Ga) DOTATATE PET/MR imaging as part of a prospective observational study (ClinicalTrials.gov Identifier: NCT04081701). Exclusion criteria included contraindications to gadolinium-based contrast agents, a history of an allergic reaction to (68Ga) DOTATATE, and pregnancy. Within this larger cohort, 14 patients were identified as having pathology-proved WHO grade 3 meningiomas and were included in our study. None of the patients met the exclusion criteria.
Clinical Annotation
Clinical chart review was performed to collect clinical and demographic characteristics of the patient population, including age, sex, surgical history, and RT history. PFS was determined from the date of the initial surgery with a diagnosis of malignant meningioma to the date of recurrence or progression by the Response Assessment in Neuro-Oncology (RANO; https://radiopaedia.org/articles/rano-criteria-for-glioma?lang=us) criteria on MR imaging, death, or last radiologic follow-up.9
Image Acquisition
PET/MR imaging was performed on a Biograph mMR scanner (Siemens) in all cases except in 1 patient who was scanned on the Signa PET/MR scanner (GE Healthcare). All PET data acquisitions started at a mean of 7 [SD, 3] minutes postinjection of 172.9 [SD, 18.4] MBq of (68Ga) DOTATATE. The PET data were continuously acquired in 3D list-mode for a total of 50 minutes and then histogrammed to a single sinogram of a timeframe of 7–57 [SD, 3] minutes postinjection. The PET data were continuously acquired in 3D list-mode for a total of 50 minutes and then histogrammed to a single sinogram of a timeframe of 7–57 [SD, 3] minutes postinjection.
All PET images were reconstructed with the default ordered subsets expectation maximization reconstruction algorithms of the manufacturer with point spread function modeling using 3 iterations and 21 (Biograph mMR) or 28 subsets (Signa). The resulting image matrix size was 344 × 344 × 127 (192 × 192 × 89) voxels with a voxel size of 2.086 × 2.086 × 2.031 mm (1.875 × 1.875 × 2.780) mm for Biograph mMR (Signa). During image reconstruction, the PET data were corrected for attenuation, scatter, randoms, normalization, dead time, decay, and frame duration using the default settings. For attenuation and scatter correction, the manufacturer’s default method and settings for estimating the MR imaging–based brain tissue attenuation map were used.
MR imaging was performed according to institutional protocol, including pre- and postcontrast sagittal 3D T1 sampling perfection with application-optimized contrasts by using different flip angle evolution (SPACE; Siemens) (TR/TE, 600–700 ms/11–19 ms, 120° flip angle, 1-mm section thickness) and postcontrast 3D T2 FLAIR (TR/TE, 6300–8500 ms/394–446 ms, 120° flip angle, 1-mm section thickness). MR imaging–based attenuation correction was obtained according to the manufacturer’s standard-of-care specifications. For patients who underwent PET/CT and MR imaging separately, the CT image set of PET/CT was subsequently registered to the postcontrast T1-weighted MR images using the rigid registration algorithm residing on a syngo.via workstation (Siemens), and the resulting transformation matrix was then applied to the PET image set to register it to the MR images.
Quantitative Image Analysis
All reconstructed PET images were initially displayed in quantitative units of becquerel/milliliter. Then the absolute maximum standard uptake value (SUV) metric was calculated at every image voxel by dividing the respective becquerel/milliliter pixel value by the ratio of the administered dose of the radiotracer in units of becquerel over the subject’s body weight (in grams) to remove the confounding effect of radiotracer dose and body weight when quantifying the (68Ga) DOTATATE uptake in every tissue. Regional absolute maximum SUV scores were subsequently extracted from a set of image pixels defining the VOIs in each PET image. Previous studies with (68Ga) DOTATATE PET have demonstrated high sensitivity and specificity in measuring cellular SSTR2 expression in the target regions with both absolute maximum SUV and SUV ratio (SUVR) normalized to the superior sagittal sinus (SSS).10 If multiple (68Ga) DOTATATE scans were available, the scan closest to the time of the initial surgery that diagnosed WHO grade 3 meningioma was used for analysis.
To standardize the comparison of lesion SUV across patients, we normalized the VOI-based lesion SUV to the SSS SUV (cranial blood pool). The VOIs were drawn for the target lesions, and maximum SUVs were reported as part of the routine clinical radiology report at our institution. The maximum SUV is referred to as SUV hereafter. The PET/MR imaging and PET/CT images were read by a fellowship-trained neuroradiologist with additional board certification in nuclear medicine. The images were interpreted for the clinical purpose of diagnosing suspected CNS SSTR2-positive tumors, and the radiologists had access to the full patient information at the time of study interpretation. The anatomic delineation of the VOIs in the PET images was based on the coregistered sagittal 3D T1-weighted postcontrast MR images with respective axial and coronal reformats, which were drawn to include the entire pituitary gland as visualized on postcontrast T1 imaging, as determined by the interpreting neuroradiologist.
Genomic and Molecular Pathology Characterization
Next-generation targeted sequencing was performed on surgically removed tumors in 11 of 14 patients using the Oncomine Comprehensive Assay. Of the 6 tumors analyzed from the secondary-progressive cohort, 1 sample was WHO grade 1, three samples were WHO grade 2, and 2 samples were WHO grade 3 at the time of genetic analysis. All 5 samples from the de novo cohort were WHO grade 3. The oncologic assay detects mutations in 99 genes, copy number variation in 49 genes, and fusion in 23 genes. TERT promoter mutations were not included in this panel. If multiple molecular pathology studies were obtained in an individual patient, only the most recent results were included for analysis.
Statistical Analysis
The Mann-Whitney test was performed to compare SUVs, surgical and radiation therapy history, and mutational burden of meningioma lesions of patients with de novo–versus–secondary-progressive tumor. PFS was analyzed by Kaplan-Meier curves, and comparisons between groups were performed using log-rank tests.
Ethics Approval and Consent to Participate
Institutional review board approval was obtained from the institutional review board committee of Weill Cornell Medicine for this Health Insurance Portability and Accountability Act–compliant study. All experimental protocols were approved by the Weill Cornell Medicine institutional review board committee. Informed consent was obtained from all subjects. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for Publication
Consent to publish any individual data has been obtained as part of the informed consent process.
RESULTS
Patient Characteristics
Fourteen patients met the inclusion criteria. The mean age was 65.6 years (range, 43–81 years), and 7 of 14 were women (50%). The average follow-up time defined by time elapsed from initial surgery diagnosing WHO grade 3 meningioma to the latest neuroradiographic follow-up was 3.6 years (range, 0.3–11.5 years). All patients had surgical resection of their meningioma, 9 of 14 required ≥2 surgeries in total, and 13 of 14 patients (93%) had adjuvant RT. Twelve of 14 patients (86%) had progression or recurrence of disease by the RANO criteria applied on follow-up MR imaging examinations post-initial surgical resection.
When we compared de novo–versus–secondary-progressive disease cohorts, 7 patients (50%) were initially diagnosed with de novo WHO grade 3 meningiomas and 7 (50%) were initially diagnosed with either WHO grade 1 or 2 and secondarily progressed to WHO grade 3 meningioma. Within the secondary-progressive cohort, 2 patients had an initial diagnosis of WHO grade 1 meningioma, while 5 patients had an initial diagnosis of WHO grade 2 meningioma. Clinical and demographic characteristics of comparing the 2 cohorts are outlined in Table 1. The secondary-progressive cohort had a significantly higher per-patient number of surgeries (4.1 versus 1.6; P = .011) and a trend toward a higher number of RT courses (2.4 versus 1.6; P = .23) and a higher cumulative RT dose (106Gy versus 68.3Gy; P = .31) during their treatment course. Gross total resection (GTR), assessed by MR imaging following initial surgery diagnosing malignant meningioma, was achieved in 86% of patients in the de novo cohort versus only 57% in the secondary-progressive cohort (P = .56). The secondary-progressive cohort had a median of 7 individual lesions per patient compared with a median of 3 individual lesions per patient identified in the de novo cohort (P = .13). Extracranial metastasis of meningioma was observed in 3 of 7 patients in the secondary-progressive cohort versus 1 of 7 patients in the de novo cohort (P = .56), with metastasis sites most commonly found in the liver but also in the lung and bones. There was no statistically significant difference in age or sex between the 2 groups.
Comparison of demographics, surgical, and radiation history, PFS, molecular pathology, and SUV on (68Ga) DOTATATE PET between patients with de novo–versus–secondary-progressive WHO grade 3 meningiomaa
Clinical Outcomes
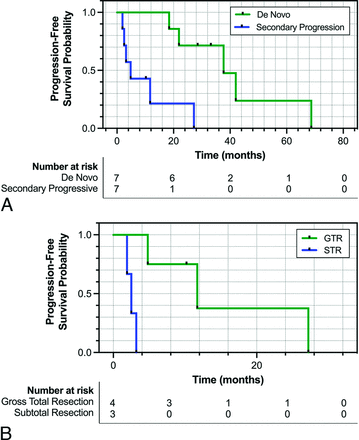
Patients in the secondary-progressive cohort had significantly lower PFS following a diagnosis of WHO grade 3 meningioma, with a median PFS of 4.8 months compared with 37.7 months observed in the de novo cohort (P = .004) (Fig 1A). Across the entire cohort, the median PFS was 20.1 months. Within the secondary-progressive cohort, those determined to have achieved GTR on postsurgical MR imaging had a significantly higher median PFS of 11.7 months compared with those with subtotal resection who had a median PFS of only 2.5 months (P = .01) (Fig 1B).
A, Kaplan-Meier curve comparing the PFS of patients with de novo (green) versus secondary-progressive (blue) WHO grade 3 meningioma. B, Kaplan-Meier curve comparing the PFS of patients with secondary-progressive WHO grade 3 meningioma who underwent GTR (green) versus subtotal resection (STR) (blue). PFS was determined using the RANO criteria on follow-up MR imaging post-initial surgery diagnosing WHO grade 3 meningioma.
Genomic Analysis
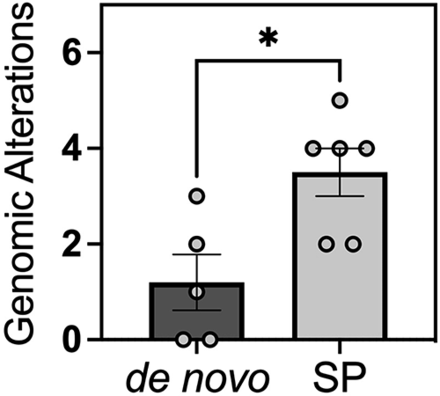
Molecular pathology profiling was performed using the Oncomine Comprehensive Assay across 5 samples in the de novo cohort as well as 6 samples from the secondary-progressive cohort. Notably, the 6 samples from the secondary-progressive cohort included 1 sample that was WHO grade 1, two samples that were WHO grade 2, and 3 samples that were WHO grade 3 at the time of genetic analysis. Despite undergoing molecular pathology profiling at lower grades, the secondary-progressive tumors exhibited a distinct molecular profile with a statistically significant higher number of mutations overall at 3.5-versus-1.2 mutations identified per tumor in the de novo cohort (P = .024) (Fig 2). The secondary-progressive malignant meningiomas were also found to have significantly higher clinically significant mutations (Table 2). Mutations identified by the Oncomine Comprehensive Assay were stratified into either pathogenic or variants of uncertain significance. When the specific mutations were stratified by clinical significance, the de novo cohort had mutations that were 33% pathogenic and 67% of unknown significance, whereas the secondary-progressive malignant meningiomas exhibited mutations that were 72% pathogenic and 28% of unknown significance (Table 2). Across the cohort, the most common mutations were in NF2 followed by CDKN2A and TP53.
Summary of molecular pathology profiling through the Oncomine Comprehensive Assay across 5 samples in the de novo cohort as well as 6 samples from secondary-progressive cohorta
Descriptive and Correlative Analysis of SUV
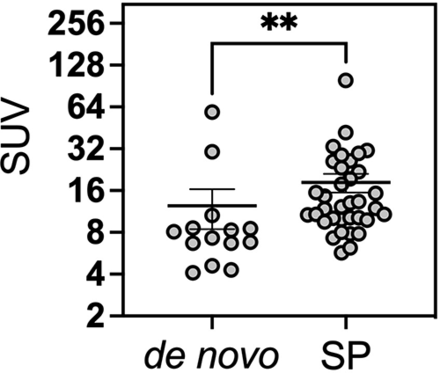
Across all lesions, the mean SUV was 15.8. (68Ga) DOTATATE PET demonstrated significantly higher SUVs in secondary-progressive tumors, with a mean SUV of 17.1 compared with 12.4 in the de novo group (P = .0021) (Fig 3). Similarly, the SUV ratio relative to the SSS tended to be higher in the secondary-progressive cohort; however, this difference did not reach statistical significance (SUVR of 14.8 versus 11.5, P = .31). Pituitary gland SUVs were not significantly different between the 2 groups (pituitary SUV of 16.3 versus 15.3 in the secondary-progressive and de novo cohorts, respectively; P = .63). SUV_SSS were not statistically different between the 2 cohorts, with SUV_SSS of 1.1 and 1.4 in the de novo and secondary-progressive cohorts, respectively (P = .17). Representative patient images from each cohort are shown in Fig 4.
Comparison of the number of genomic alternations per tumor between de novo versus secondary-progressive (SP) WHO grade 3 meningiomas. The Mann-Whitney test was performed to test statistical significance. A single asterisk indicates P value < .05.
SUV on (68Ga) DOTATATE PET/MR of de novo–versus–secondary-progressive (SP) WHO grade 3 meningioma lesions. The Mann-Whitney test was performed to test the statistical significance. Double asterisks indicate P value < .01.
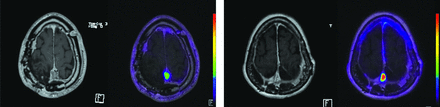
Representative cases of DOTATATE-PET/MR imaging and corresponding T1-weighted postcontrast images of a patient with de novo WHO grade 3 meningioma (left) and a patient with secondary-progressive WHO 3 meningioma (right).
DISCUSSION
While meningiomas overall are the most common primary brain tumor, WHO grade 3 meningiomas are rare and thus understudied, with only around 300 cases of WHO grade 3 meningiomas diagnosed annually in the United States, limiting our understanding of the natural history as well as the optimal diagnostic and therapeutic management.11 While patients with WHO grade 3 meningiomas are typically treated with maximal surgical resection and RT, clinical outcomes are poor, with recurrence rates of 50%–90% by 5 years after surgery and a 5-year overall survival rate of 20%–50%.1⇓-3 Given their high morbidity and mortality rates, it is critical to optimize the diagnostic and therapeutic management of WHO grade 3 meningiomas.
Whether WHO grade 3 meningiomas arise de novo or from secondary progression has also proved to have important prognostic implications. Approximately one-half of all WHO grade 3 meningiomas are diagnosed de novo, while the other half arise from secondary progression of a previously diagnosed lower histologic grade. In our study, patients with secondary-progressive WHO grade 3 meningiomas had markedly decreased PFS compared with the de novo cohort, consistent with previous findings from other studies.2,4 The exact mechanism underlying this observation is not yet clearly understood, though 1 explanation may lie in the differences in molecular pathology.
Although it is known that higher-grade meningiomas have greater rates of genetic mutations, we found that even within WHO grade 3 meningiomas, those that progressed from lower-grade tumors compared with de novo cases had a distinct molecular profile with higher rates of genetic mutations overall and in specific genes such as NF2 and CDKN2A/B, which have been known to be implicated in more aggressive disease reported in the literature.12 Our genetic data were collected through the Oncomine Comprehensive Assay, which is a targeted next-generation sequencing assay that tests a panel of genes with known oncologic implications, but it notably did not include TERT promoter mutations. For the secondary-progressive cohort, genomic data were available from 6 patients; however, some samples were analyzed before dedifferentiation to WHO grade 3, with 1 sample being WHO grade 1 and 2 samples being WHO grade 2 at the time of molecular testing. Nevertheless, the secondary-progressive cohort still had a greater mutational burden both in number and clinical relevance compared with the de novo cohort. This finding may help to explain the more aggressive clinical course of secondary-progressive WHO grade 3 meningiomas, arguing that de novo–versus–secondary-progressive status may be an important prognostic factor for WHO grade 3 meningiomas, and they may even be considered 2 distinct clinical entities moving forward.
MR imaging, the criterion standard for meningioma surveillance, has significant limitations such as distinguishing recurrent or residual disease from postoperative findings, and there has been a marked unmet need for more targeted imaging strategies, especially in intermediate- and high-risk tumors.6 More recently, (68Ga) DOTATATE PET has demonstrated high utility in meningioma evaluation, including diagnostic confirmation, surgical planning, delineation of radiation therapy target volumes, and posttreatment surveillance.7 Although the utility of (68Ga) DOTATATE PET/MR has been shown in meningioma diagnosis and treatment-planning, its role has not previously been systematically investigated in patients with WHO grade 3 meningioma, in part due to limited sample sizes. This is the first study evaluating the clinical characteristics and utility of (68Ga) DOTATATE PET/MR imaging in a cohort of patients with exclusively WHO grade 3 meningioma and comparing secondary-progressive and de novo cohorts. Just as seen previously with WHO grades 1 and 2 meningiomas, (68Ga) DOTATATE PET is very useful in distinguishing recurrent or residual disease from postoperative findings in WHO grade 3 meningioma compared with evaluation on MR imaging alone. For instance, 1 patient with surgical resection of WHO grade 3 meningioma was thought to have achieved GTR on postsurgical MR imaging with questionable residual disease versus postoperative changes but then later was found to have had definitive residual disease and subtotal resection on (68Ga) DOTATATE PET.
Previously, (68Ga) DOTATATE has been routinely used in the evaluation of gastroenteropancreatic neuroendocrine tumors years before it was used in meningioma management. For gastroenteropancreatic neuroendocrine neoplasms, the sensitivity of (68Ga) DOTATATE decreases with an increased grade of the tumor, possibly due to downregulation of SSTR2 with increasing tumor grade.13 A similar mechanism in meningiomas could be hypothesized; however, we found that on the contrary, (68Ga) DOTATATE PET SUV was significantly higher in secondary-progressive WHO grade 3 tumors compared with de novo tumors, despite the secondary-progressive tumors having significantly higher rates of genetic mutations. Our findings suggest that downregulation of SSTR2 does not occur with an increase in WHO grade in meningiomas, with the possibility that the receptor may be upregulated. Thus, (68Ga) DOTATATE remains a clinically useful approach in meningiomas regardless of WHO grade.
At present, determining meningioma grade is not possible on the basis of neuroimaging alone. Our findings suggest the possibility of noninvasively differentiating secondary-progressive from de novo WHO grade 3 meningiomas using (68Ga) DOTATATE PET/MR imaging, which has high clinical relevance, given the known difference in prognosis between the 2 groups previously shown by other authors and confirmed in our study.
Additionally, this work raises important questions regarding the role of SSTR2 in meningioma biology. SSTR2 downstream signaling is implicated in a diverse range of physiologic processes such as the secretion of insulin and glucagon, thyroid-stimulating hormone and growth, and neuronal excitability.14,-,16 However, the role of SSTR2 in meningioma biology and the correlation between SSTR2 expression and clinical outcomes remain to be determined prospectively. In immunohistochemical studies, no correlation between SSTR2 expression and tumor grade was found; however, there was a positive correlation with higher microvessel density.17,18 Clinically, higher SSTR2 expression was correlated with a higher risk of recurrence after surgical resection.18,19 Notably, higher SSTR2 expression was correlated with improved PFS following somatostatin-receptor (SSTR)-targeted radionuclide therapy, which may be due to higher binding of the SSTR-targeted theragnostic agent and thus higher treatment effectiveness.18,19
Taken together, it is still unclear whether SSTR2 expression is related to tumor grade or prognosis. Our data suggest that secondary-progressive WHO grade 3 meningiomas have an elevated (68Ga) DOTATATE SUV compared with de novo disease, suggesting a link between elevated SSTR2 expression and worsened clinical outcomes, though formal immunohistochemical studies have yet to be performed. Notably, in a study encompassing all WHO grade meningiomas, a higher DOTATATE PET SUV was associated with a higher tumor growth rate,20 which is concordant with our WHO grade 3–specific findings, with higher SUVs reflecting worsened PFS. However, in our study, we were unable to directly correlate tumor SUV and PFS due to the small sample size. In another study, there was a strong correlation between tumor vascularity and SSTR2 expression in WHO grades 2 and 3 but not grade 1 meningiomas, suggesting that SSTR2 may play a role in tumor vascularity in higher-grade tumors.21
SSTR2 expression in meningiomas has also emerged as a potential therapeutic target. SSTR2-directed peptide receptor radionuclide therapy with β-emitters 90 yttrium (90Y) and 177 lutetium (177Lu) has shown promise in the treatment of unresectable or refractory meningiomas.22,23 Other systemic treatment options have emerged such as dacarbazine and adriamycin, hydroxyurea, temozolomide, and irinotecan, but their efficacy has not yet been shown in clinical trials.24 While rare, SSTR2-negative meningioma has been reported in the literature and is an important additional indication of a lack of direct correlation between SSTR2 expression and WHO grade.25 SSTR2-targeted therapeutic effort may be beneficial in WHO grade 3 meningiomas, possibly more so in the secondary-progressive cohort, which may be expressing higher levels of SSTR2.
One significant limitation of this study is the determination of de novo disease, because it is possible that tumors arose from lower-grade meningiomas but were only first diagnosed with pathology once the tumor had already progressed to WHO grade 3 disease. Although this limitation may be an unavoidable confounder in the original categorization of WHO grade 3 meningiomas as either being de novo or secondary-progressive, our data suggest that regardless of de novo–versus–secondary-progressive status, those with a higher SUV, on average, had shortened PFS following surgical resection. This finding suggests that in WHO grade 3 meningiomas, SUV alone could be predictive of worse prognosis and thus guide clinical decision-making. Another limitation to our study is the small sample size; however, due to the low incidence of the disease, this has been the case in all other published studies and remains a bottleneck in our effort to fully understand the natural history and optimal management of WHO grade 3 meningiomas. Nevertheless, our study remains the first to investigate a cohort of WHO grade 3 meningiomas with (68Ga) DOTATATE PET/MR imaging and thus has the potential to increase our understanding of this rare entity, thereby improving clinical outcomes.
CONCLUSIONS
This is the first study evaluating the clinical characteristics and utility of (68Ga) DOTATATE PET/MR imaging in a cohort of patients with WHO grade 3 meningiomas and comparing secondary-progressive and de novo WHO grade 3 tumors. We found that compared with the de novo group, the secondary-progressive cohort demonstrated worsened clinical outcomes, including significantly decreased PFS, in accordance with previous studies. In addition to distinct molecular profiles with higher mutational burden, we also report significantly increased SUVs in secondary-progressive compared with de novo WHO grade 3 meningiomas. This work further supports (68Ga) DOTATATE PET/MR imaging as a useful management strategy in WHO grade 3 meningiomas and raises the possibility of differentiating secondary-progressive and de novo malignant meningiomas with PET/MR imaging in the clinical context. This work raises important questions regarding meningioma biology such as the potential role of SSTR2 signaling in WHO grade 3 meningiomas, which may represent a potential therapeutic target in future clinical trials.
Acknowledgments
We thank the patients and their families for their contributions to the study and especially Ms Alexis Watson and Ms Eileen Chang for their help with research coordination and administrative support.
Footnotes
Paper previously presented as an abstract at: Annual Meeting of the Radiological Socity of North America, November 26–30, 2023. Chicago, Illinois.
The study was partially funded by an investigator-initiated clinical trial grant (funder: Novartis Pharmaceuticals CAAA501A0US05T to J.I.).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received December 15, 2023.
- Accepted after revision February 2, 2024.
- © 2024 by American Journal of Neuroradiology