Abstract
SUMMARY: Multisystem inflammatory syndrome in children is a recently described complication in the late phase of Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) infection involving systemic hyperinflammation and multiorgan dysfunction. The extent of its clinical picture is actively evolving and has yet to be fully elucidated. While neurologic manifestations of SARS-CoV-2 are well-described in the adult population, reports of neurologic complications in pediatric patients with SARS-CoV-2 infection are limited. We present a pediatric patient with SARS-CoV-2 infection with development of multisystem inflammatory syndrome and acute encephalopathy causing delirium who was found to have a cytotoxic lesion of the corpus callosum on neuroimaging. Cytotoxic lesions of the corpus callosum are a well-known, typically reversible entity that can occur in a wide range of conditions, including infection, seizure, toxins, nutritional deficiencies, and Kawasaki disease. We hypothesized that the cytotoxic lesion of the corpus callosum, in the index case, was secondary to the systemic inflammation from SARS-CoV-2 infection, resulting in multisystem inflammatory syndrome in children.
ABBREVIATIONS:
- CLOCC
- cytotoxic lesion of the corpus callosum
- COVID-19
- coronavirus disease 2019
- IVIG
- intravenous immunoglobulin
- MIS-C
- multisystem inflammatory syndrome in children
- PCR
- polymerase chain reaction
- SARS-CoV-2
- Severe Acute Respiratory Syndrome coronavirus 2
Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) infection in the pediatric population is not well-understood and has, until recently, been thought of as a relatively mild disease compared with that in adults. Most reported symptoms in children are fever, respiratory tract infection, or asymptomatic infection.1 More recent reports in the pediatric population, however, describe a multisystem inflammatory syndrome in children (MIS-C).2⇓⇓⇓-6 In the adult population, neurologic manifestations of coronavirus disease 2019 (COVID-19) are being reported with increasing frequency, including seizure, Guillain-Barré syndrome, vasculitis, stroke, cranial nerve palsy, and general encephalopathy.7⇓⇓⇓-11 Cases of neurologic manifestations in pediatric patients have been limited.
We report the case of a pediatric patient positive for COVID-19 who presented with fever, vomiting, diarrhea, cough, difficulty walking, and delirium.
Case Presentation
A 13-year-old previously healthy girl, who relocated from New York to Georgia a month prior, presented to the emergency department after 3 days of fever, vomiting, diarrhea, cough, dizziness, and gait instability. The only medications given before admission were aspirin and ibuprofen. On presentation to Egleston Hospital of Children's Healthcare of Atlanta, she had a fever of 39.2°C, hypotension initially requiring pressor support, normal white blood cell count (9780 cells/µL) with increased neutrophils (88%) and lymphopenia (7%), thrombocytopenia (121,000 cells/µL), hyponatremia (128 mmol/L), elevated transaminases (aspartate aminotransferase = 292 U/L, alanine aminotransferase = 336 U/L), acute kidney injury (creatinine = 1.09 mg/dL), elevated inflammatory markers (C-reactive protein = 10.9 mg/dL, erythrocyte sedimentation rate = 63 mm/h, interleukin 6 = 65 pg/mL), and elevated cardiac markers (troponin = 18.2 ng/mL, brain natriuretic peptide = 561 pg/mL). She was admitted to the pediatric intensive care unit for presumed bacterial sepsis and started on ceftriaxone and vancomycin.
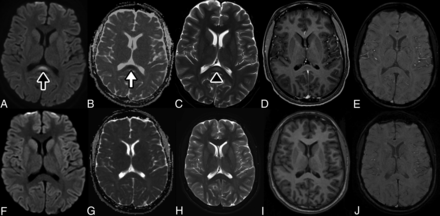
On hospital day 1, she began to have hallucinations in addition to urinary retention, prompting neurology consultation. On neurologic examination, she was agitated and combative with a variable rate of speech. She had intermittent auditory hallucinations. Her attention fluctuated. She was able to follow 1- but not 2-step directions. Cranial nerve function was normal without clinical evidence of anosmia. Muscle tone was decreased with symmetric antigravity movement of all extremities. Sensation to light touch was intact throughout. Deep tendon reflexes were 1+ with absent ankle jerks. Contrast-enhanced MR imaging of the brain (Magnetom Avanto, 1.5T; Siemens) on hospital day 1 demonstrated an abnormal focal nonenhancing lesion with increased intensity on T2-weighted sequences and restricted diffusion in the splenium of the corpus callosum (Figure). Findings on MR imaging of the spine were normal. CSF studies were unremarkable (4 white blood cells (WBC)/μL, glucose = 83 mg/dL, protein = 32 mg/dL). The electroencephalogram showed diffuse slowing without epileptiform discharges or seizures. On hospital day 2, the patient developed tachypnea requiring escalation of respiratory support to bilevel positive airway pressure, though no desaturation or hypoxemia was noted. The echocardiogram revealed depressed cardiac function with a left ventricular ejection fraction of 38%. Intravenous immunoglobulin (IVIG), 2 g/kg, was administered on hospital day 2, given concerns for atypical Kawasaki-like multisystem inflammatory state.
CLOCC in an adolescent with MIS-C and SARS-CoV-2.. MR image of the brain demonstrates a nonspecific focus of increased signal in the splenium of the corpus callosum on DWI sequences at b=1000 s/mm2 (black arrow, A) with associated loss of signal on apparent diffusion coefficient maps (white arrow, B) corresponding to restricted diffusion. The apparent diffusion coefficient for the lesion is 0.44 × 10−3 mm2/s. This lesion corresponds to a faint focus of abnormal increased signal on T2WI spin-echo sequences (black arrowhead, C). The lesion also demonstrates a lack of contrast enhancement on T1WI postcontrast thin-section image (D) and absent susceptibility, suggesting absent hemorrhage on SWI (E). The imaging findings suggest a cytotoxic lesion of the corpus callosum. Follow-up DWI (F) and ADC maps (G) with T2WI (H), T1WI (I), and SWI sequences (J) after 2.5 months demonstrate resolution of the lesion after therapy.
Extensive infectious and autoimmune investigations were performed. Respiratory viral panel and initial SARS-CoV-2 polymerase chain reaction (PCR) findings on admission were negative. The remainder of infectious evaluation was negative (CSF meningitis panel, CSF and serum arboviral panels, serum viral studies including coxsackie A9 antibody, West Nile virus antibodies, human immunodeficiency virus antigen and antibody, enterovirus PCR, parvovirus B19 PCR, adenovirus PCR, and Epstein-Barr virus PCR). The serum autoimmune encephalopathy panel had a mildly elevated glutamic acid decarboxylase antibody (0.17 nmol/L) in the setting of recent IVIG administration and was thought to likely be a false-positive. Antinuclear antibody titer was elevated to 1:320 with a negative antibody reflex panel. The remainder of autoimmune work-up had negative findings (CSF oligoclonal bands, immunoglobulin G index, CSF autoimmune encephalopathy panel, serum thyroglobulin and antithyroid peroxidase antibodies, myelin oligodendrocyte glycoprotein antibody, aquaporin-4 antibody). Given the high level of suspicion, SARS-CoV-2 PCR was repeated on hospital day 3 and found to be positive. Qualitative COVID-19 immunoglobulin G in serum drawn on hospital day 2 before IVIG administration was also positive.
During the next few days, the patient’s mental status improved gradually; however, she remained weak and tendon reflexes were persistently absent in the ankles. Creatine kinase was elevated (3600 U/L, normal = 50–275 U/L), and the electrodiagnostic study performed on hospital day 5 was unremarkable with no evidence of polyneuropathy or myopathy. On hospital day 6, respiratory support was weaned to nasal cannula, and she was able to stand unassisted. The repeat echocardiogram demonstrated return to normal cardiac function, and laboratory markers normalized. She was transferred out of the intensive care unit and discharged home 12 days following the initial presentation with follow-up in the pediatric neurology clinic scheduled. Furthermore, subsequent research laboratory studies on the CSF did not detect the presence of SARS-CoV-2 by PCR. An indirect enzyme-linked immunosorbent assay for CSF showed increased levels of immunoglobulin M (1:64) for SARS-CoV-2 S1 and E (envelope) proteins (90% sensitivity, 89% specificity; courtesy of the Dr. William Hu Laboratory, Emory University).
DISCUSSION
We describe a pediatric case of a cytotoxic lesion of the corpus callosum (CLOCC) in the setting of MIS-C due to SARS-CoV-2 infection. CLOCCs have been well-described in a wide variety of conditions, including infection, seizure, toxins, nutritional deficiencies, and Kawasaki disease.12,13 Clinically, these lesions can manifest in a wide variety of nonspecific symptoms, such as cognitive impairment, seizures, hallucinations, delirium, dysarthria, and motor weakness.12,13 Clinically, the CLOCC may have contributed to her acute encephalopathy, causing delirium with prominent auditory hallucinations. MR imaging features of CLOCCs include increased signal intensity on FLAIR sequences and decreased signal intensity on T1-weighted sequences. Diffusion is reduced, and there is no contrast enhancement.13
CLOCCs associated with classic Kawasaki disease are a well-known entity. In hyperinflammatory states, macrophage activation leads to cytokine release that subsequently results in T-cell recruitment and breakdown of the blood-brain barrier. As the cytokine cascade invades the CNS, astrocytes are triggered to release excess glutamate, precipitating further cytokine release from microglia. The corpus callosum is especially vulnerable to this attack due to its high concentration of cytokine and glutamate receptors.13 In the index case, a CSF cytokine assay is pending.
SARS-CoV-2 has recently been demonstrated to result in MIS-C.2⇓⇓⇓-6 This post or peri-infectious immune-mediated disorder leads to persistent fever, gastrointestinal symptoms, lymphadenopathy, and left-heart dysfunction often requiring inotropic support, such as seen in Kawasaki disease.3
The study patient developed MIS-C characterized by elevated serum interleukin 6 and cardiac enzymes, acutely reduced cardiac function, and neurologic dysfunction with neuroimaging showing CLOCC. Most interesting, this is similar to previously reported Kawasaki disease associated with CLOCCs.12 We hypothesize that the SARS-CoV-2 postor peri-infectious immune-mediated disorder leads to MIS-C with neurologic dysfunction and CLOCC. This concept is further supported by absent SARS-CoV-2 PCR findings and elevated cytokines in the CSF as shown by Benameur et al.14 In this case, clinical improvement was noted with supportive care and IVIG. Repeat MR imaging 3 months following the initial illness demonstrated resolution of CLOCC, with no residual signal abnormality.
Reports continue to emerge of severe manifestations of COVID-19 in children, despite previous reports of mild illness. This case of MIS-C associated with COVID-19 highlights the potential of MR imaging abnormalities resulting in acute encephalopathy causing delirium and emphasizes the need for careful consideration of possible neurologic complications of SARS-CoV-2 infection.
Acknowledgments
We thank the patient and her family along with the physicians and nurses who provided clinical excellence and compassionate care.
Footnotes
J. Lin and E.C. Lawson contributed equally as co-first authors.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- Received June 2, 2020.
- Accepted after revision July 8, 2020.
- © 2020 by American Journal of Neuroradiology