Abstract
BACKGROUND AND PURPOSE: The implications of basal ganglia T1 hyperintensity remain unclear in patients with hereditary hemorrhagic telangiectasia. This study was performed to assess the prevalence of this imaging finding in a large cohort of patients with hereditary hemorrhagic telangiectasia and to identify any association between this phenomenon and other disease manifestations.
MATERIALS AND METHODS: In this retrospective study, we identified all patients at our institution diagnosed with definite hereditary hemorrhagic telangiectasia from 2001 to 2017. Patients who did not undergo brain MR imaging were excluded. Patient demographics, laboratory results, and hereditary hemorrhagic telangiectasia disease characteristics were noted. Basal ganglia hyperintensity was evaluated both qualitatively and quantitatively relative to the signal intensity in the ipsilateral thalami. Statistical analysis was performed with commercially available software.
RESULTS: A total of 312 patients (41% men, 59% women; mean age, 51 ± 18 years) with definite hereditary hemorrhagic telangiectasia were identified. Basal ganglia T1 hyperintensity was present in 23.4% of patients and demonstrated a statistically significant association with older age (P < .001), increased hepatic AVMs (P < .001), high cardiac output state (P < .001), hepatic failure (P = .01), elevated peak serum alkaline phosphatase level (P = .03), and increased total bilirubin count (P = .03). There was no significant association with sex, hereditary hemorrhagic telangiectasia genetic mutation status, parkinsonism, or serum transaminase levels.
CONCLUSIONS: Basal ganglia T1 hyperintensity occurs in >23% of patients with hereditary hemorrhagic telangiectasia and is associated with hepatic vascular malformations, hepatic dysfunction, and elevated cardiac output. The presence of this finding on screening MR imaging in patients with hereditary hemorrhagic telangiectasia should prompt further evaluation for visceral lesions causing arteriovenous shunting.
ABBREVIATIONS:
- ALT
- alanine aminotransferase
- ALK1
- activin receptor-like kinase 1
- AST
- aspartate aminotransferase
- ENG
- endoglin
- HHT
- hereditary hemorrhagic telangiectasia
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant vascular disorder characterized by arteriovenous malformations in multiple visceral and mucocutaneous vascular beds. The Curaçao diagnostic criteria include the following: 1) the presence of spontaneous and recurrent epistaxis, 2) mucocutaneous telangiectasias, 3) visceral arteriovenous malformations, and 4) a first-degree relative diagnosed with HHT using the same criteria.1 Patients who meet at least 3 criteria are given a definite diagnosis of HHT, while those meeting 2 criteria are diagnosed with possible or suspected HHT; patients meeting <2 criteria are considered unlikely to have HHT. Siblings and children of affected patients have a 50% risk of inheriting the disorder.2
The most common clinical presentation of HHT is epistaxis early in the second decade of life.2 In hospitalized patients, epistaxis and gastrointestinal hemorrhage occur in approximately 16% and 11% of patients, respectively, while congestive heart failure occurs in nearly 20% of patients.3 Visceral arteriovenous malformations predominantly involve the liver, lungs, and brain, and their prevalence is related to the specific underlying mutation.4 Patients with mutations in the endoglin (ENG) gene (HHT-1) more often have cerebral and pulmonary AVMs, while patients with mutations in the activin receptor-like kinase 1 (ALK1) gene (HHT-2) are more likely to have hepatic AVMs; the rare SMAD family member 4 mutation is associated with juvenile colonic polyposis.5,6 The overall prevalence of cerebral AVMs is 10%, with a higher prevalence in HHT-1 versus HHT-2 (13.4% versus 2.4%).7 Although clinically silent in nearly 45% of patients, AVMs may cause substantial morbidity, depending on their location, size, and the degree of arteriovenous shunting. Baseline brain MR imaging screening is recommended to minimize the risk of potentially fatal complications from cerebral AVMs.8
Some patients with HHT demonstrate abnormally increased basal ganglia signal intensity on T1-weighted MR images of the brain.9⇓–11 This finding has been attributed to intracranial deposition of paramagnetic manganese in hepatic arteriovenous shunting by hepatic AVMs.11 However, the incidence and implications of this imaging finding remain unclear in the HHT population. Therefore, we conducted this study to assess the prevalence of basal ganglia T1 hyperintensity in a large cohort of patients with HHT and to identify any association between this phenomenon and other disease manifestations.
Materials and Methods
Patient Population
This retrospective, single-institution study was approved by our institutional review board with a waiver of written informed consent and was performed in compliance with the Health Insurance Portability and Accountability Act. An initial patient list was generated by searching the institutional data base for all patients with HHT between January 2001 and January 2017. Inclusion criteria were the following: 1) patients with a definite diagnosis of HHT as defined by the Curaçao criteria, and 2) patients who underwent 1.5T or 3T MR imaging with at least 1 noncontrast T1-weighted scan. Patients without MR imaging of the brain were excluded from the study cohort.
Imaging Analysis
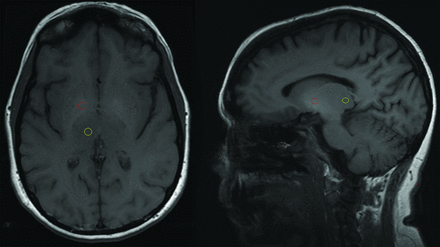
Basal ganglia hyperintensity was qualitatively determined relative to the signal intensity in the ipsilateral thalami. Quantitative measurements of signal intensity were obtained from 4- to 6-mm ovoid ROIs manually placed on the bilateral lentiform nuclei and thalami on axial or sagittal unenhanced T1-weighted images of the brain (Fig 1). In patients who underwent multiple MRIs, the earliest available scan was used to exclude gadolinium deposition as a cause for the increased T1 signal. The clinical record and relevant imaging were scrutinized to assess for potential confounders such as calcifications, toxins, hemorrhage, or ischemia.
Axial and sagittal T1-weighted MR images of the brain demonstrating placement of ovoid ROIs on the basal ganglia (red) and ipsilateral thalamus (yellow).
Data Collection
The electronic medical record was reviewed to obtain patient demographics, laboratory values, and disease characteristics. Demographic information included age and sex. Laboratory studies disclosed the following values: total serum bilirubin, alkaline phosphatase, alanine aminotransferase, and aspartate aminotransferase, as well as cardiac output. HHT disease characteristics included the presence of mucocutaneous telangiectasias, epistaxis, family history, and genetic mutation status. Data regarding liver failure, dyskinesias, or clinical parkinsonism (tremor, bradykinesia, rigidity, and postural instability) were also collected. Relevant imaging was reviewed on the PACS to identify hepatic, pulmonary, and cerebral arteriovenous malformations (dysplastic vascular arterial and venous communication without an intervening capillary bed).
Patients were divided into 2 groups based on the presence or absence of basal ganglia hyperintensity, and the groups were compared with regard to the above-mentioned variables.
Statistical Analysis
Statistical analysis was performed with commercially available software (JMP 7; SAS Institute, Cary, North Carolina). Continuous variables were compared using the Student t test. The χ2 test was used to compare categoric variables. P values < .05 were considered statistically significant.
Results
Patient Population and Imaging Analysis
A total of 312 patients (41% men, 59% women; mean age, 51 ± 18 years) were included in the study cohort. A total of 73 patients had qualitatively bright basal ganglia on imaging analysis, and 239 patients had normal-appearing basal ganglia. The mean basal ganglia/thalamic ratio for the bright basal ganglia group versus the normal basal ganglia group was 1.27 ± 0.14 versus 1.07 ± 0.07 on the right side (P < .001) and 1.26 ± 0.13 versus 1.07 ± 0.07 on the left side (P < .001). Patient demographic and disease characteristics are summarized in Table 1. Representative cases are demonstrated in Figs 2 and 3.
Patient demographic and disease characteristicsa
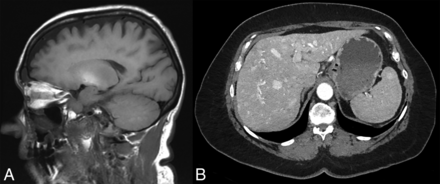
A representative patient with HHT and increased basal ganglia signal intensity on sagittal T1-weighted MR imaging (A) with a concurrent hyperenhancing hepatic AVM on arterial phase abdominal CT (B).
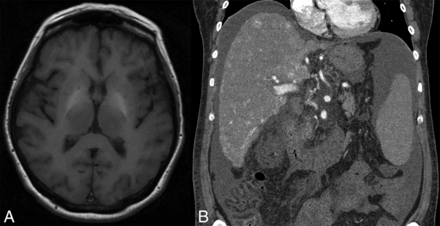
Another representative patient with HHT and increased basal ganglia signal intensity on axial T1-weighted MR imaging (A) with multiple hyperenhancing hepatic AVMs on coronal arterial phase abdominal CT (B).
Variables Associated with Basal Ganglia Hyperintensity
The presence of basal ganglia T1 hyperintensity demonstrated a statistically significant association with increased age (62 versus 48 years, P < .001), the presence of hepatic AVMs (49.3% versus 24.2%, P < .001), hepatic telangiectasias (23.3% versus 20.9%, P < .001), nasal telangiectasias (8.2% versus 2.1%, P = .02), increased peak cardiac output (7.9 versus 6.1 L/min, P < .001), hepatic failure (5.4% versus 0.8%, P = .01), and elevated peak serum alkaline phosphatase levels (148 versus 108 U/L, P = .03) and elevated total bilirubin levels (0.88 versus 0.66, P = .03). There was no significant association with sex (P = .62), genetic mutation status (P = .07), parkinsonism (1.4% versus 1.3%, P = .38), or peak serum levels of alanine aminotransferase (ALT) (91 versus 53 U/L, P = .35) or aspartate aminotransferase (AST) (166 versus 51, P = .28). These findings are summarized in Table 2.
Variables associated with BG hyperintensitya
Discussion
In this study, basal ganglia T1 hyperintensity was present in >23% of patients with definite HHT. There was a statistically significant association between this imaging finding and increased patient age, hepatic vascular malformations, hepatic dysfunction, and elevated cardiac output. There was no significant association with sex, genetic mutation status, cerebral AVMs, or gross neuropsychiatric derangement. These findings are in accordance with the prevailing hypothesis that intracranial deposition of paramagnetic manganese in the presence of hepatic AVMs is responsible for basal ganglia hyperintensity, a phenomenon that has been described in other disease processes in which hepatic metabolism of manganese is impaired or bypassed.12 Moreover, these findings are important because they suggest that the degree of shunting required to produce basal ganglia hyperintensity appears to be associated with hepatic dysfunction and increased cardiac output, both of which may entail further metabolic derangements and a worse prognosis.
The prevalence of basal ganglia hyperintensity in the present investigation was less than the rate described in a recent study of 139 patients (23% versus 38%); this prevalence may reflect the lower overall rate of hepatic AVMs in our cohort (30% versus 45%).13 This could be due to a difference in the prevalence of ENG versus ALK1 mutations in each study population, with hepatic AVMs and the high cardiac output state being much more common in those with the ALK1 mutation. Older age, higher cardiac output, and increased prevalence of hepatic dysfunction in the bright basal ganglia group merit further discussion. Hepatic vascular involvement in HHT results in shunting involving portal vein to hepatic vein, hepatic artery to portal vein, or hepatic artery to hepatic vein. It is the hepatic artery–hepatic vein shunting that results in a high cardiac output state. A recent large nationwide study of patients with HHT in the United States found that most (∼75%) patients with a high cardiac output state were older than 60 years of years of age.3 Similarly, almost 60% of patients with cirrhosis were also older than 60 years of age in that study. Thus, the finding of high cardiac output and liver dysfunction among older patients in our study in the bright basal ganglia group is not a surprise.
Predicting liver disease in HHT is an important topic because substantial liver disease can often be present in otherwise asymptomatic patients. In a multivariate prediction model, Singh et al14 reported that increasing age, female sex, higher alkaline phosphatase levels, and lower hemoglobin levels were all predictive of clinically significant liver disease among patients with HHT. Our study confirms these findings, with older age and higher alkaline phosphatase levels being more common in the bright basal ganglia group. Thus, it appears that bright basal ganglia occur more frequently in the same group of patients previously shown to have higher rates of clinically significant HHT-related liver disease. These congruent findings further validate our study hypothesis and results.
Manganese deposition has been associated with neurotoxicity in certain at-risk populations. In a recent study of workers with occupational exposure to manganese, Shin et al15 found a dose-response relationship between T1 signal intensity and serum manganese levels and further described an inverse relationship between signal and neurobehavioral performance, positing that signal intensity may predict performance in workers exposed to the element. In the present study, increased basal ganglia signal intensity was not associated with gross neurologic disturbances, though subclinical neurologic dysfunction may have existed at the time of imaging. Given the absence of overt neurologic manifestations, however, the association between basal ganglia T1 hyperintensity and liver dysfunction and elevated cardiac output highlights the importance of screening MR imaging.
Current guidelines recommend Doppler sonography or CT screening of adult patients with abnormal hepatic enzymes or clinical features suggestive of hepatic vascular malformations (ie, high output cardiac failure, liver failure, intestinal ischemia, or hepatic encephalopathy).16 Our findings suggest that basal ganglia hyperintensity on screening brain MR imaging may be the only evidence of a subclinical hepatic AVM. In this setting, it may be appropriate to raise this possibility and recommend additional imaging and diagnostic studies to determine the presence of liver AVMs along with hepatic dysfunction and a high cardiac output state. Each of these entities worsens prognosis in patients with HHT and requires specific decisions with regard to follow-up and treatment options.2 Most important, early detection of potentially consequential yet preclinical hepatic vascular malformations may facilitate timely intervention and ultimately lead to improved patient outcomes.
Limitations
Limitations of this study include its retrospective nature and the inherent weaknesses of nonprospective studies as well as the lack of formal neuropsychological assessment and lack of a corelation between basal ganglia deposits of manganese and serum manganese levels. Additionally, data were obtained during 2 decades from at least 15 different scanners and were therefore subject to variations in MR imaging technology and protocols and scan parameters; information pertaining to data obtained at 1.5T versus 3T was not available. The strengths of our study include the use of a well-characterized cohort of patients with HHT from a high-volume HHT center of excellence. In addition, each scan was carefully reviewed by a core group of experienced neuroradiologists with considerable experience in the field of HHT. Also, the availability of laboratory values and echocardiogram data from the electronic medical record allowed excellent statistical correlations to be made with increased basal ganglia T1 signal intensity.
Conclusions
Basal ganglia T1 hyperintensity due to manganese deposition occurs frequently in patients with HHT and was seen in >23% of this cohort. It was associated with older age and the presence of hepatic vascular malformations, hepatic dysfunction, and a high cardiac output state. The presence of this finding in patients with definite HHT should prompt a thorough evaluation for visceral AVMs and any associated systemic consequences.
Footnotes
Disclosures: Vivek Iyer—UNRELATED: Consultancy: Merck Pharmaceuticals, Comments: related to my work in chronic cough (site Principal Investigator for a phase 3 study); Royalties: Mayo Clinic Internal Medicine Board Review and Mayo Clinic Interventional Cardiology Board Review, Comments: I have authored a few chapters for these books and receive about US $100 a year in royalties.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- Received March 26, 2017.
- Accepted after revision May 28, 2017.
- © 2017 by American Journal of Neuroradiology