Abstract
SUMMARY: We report a case of DDL with an osteosarcomatous component affecting the right neck. CT showed a lipomatous region with thick septa, a low-attenuation high-water-content component, and a sporadic heterogeneous high-attenuation calcified area.,
Abbreviations
- DDL
- dedifferentiated liposarcoma
- HE
- hematoxylin-eosin
- WDL
- well-differentiated liposarcoma
Liposarcoma is one of the most common soft-tissue sarcomas. It is currently classified into 5 subtypes: well-differentiated, dedifferentiated, myxoid, pleomorphic, and mixed.1 DDL represents a biphasic neoplasm, with 1 component being a WDL and the other a nonadipose cellular sarcoma. The most common dedifferentiated component is undifferentiated pleomorphic sarcoma or fibrosarcoma. Dedifferentiation to osteosarcoma is rare, and only 8 cases, to our knowledge, have been described in the literature.2–9
We report a case of a young woman who had DDL of the neck with an osteosarcomatous component, to illustrate the imaging and pathologic findings.
Case Report
A 20-year-old woman presented with a painless mass in the right neck, which had been progressively enlarging for approximately 2 months. On physical examination, the right neck was markedly larger than the left. An oral examination revealed a submucosal bulging of the right lateral pharyngeal wall. The overlying mucosa appeared to be normal. The patient denied dysphagia or bucking (choking cough). The other physical examination findings were fairly unremarkable.
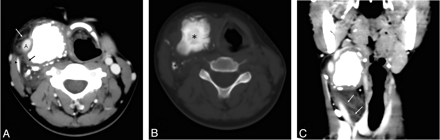
A contrast-enhanced CT scan of the head and neck revealed a large neck mass that had 3 components of differing attenuation, including a lipomatous region with thick septa, a low-attenuation high-water-content component, and a sporadic heterogeneous high-attenuation calcified area (Fig 1). After infusion of the contrast material, no enhancement was seen (Fig 1). The mass had well-defined margins between the sternocleidomastoideus muscle and the lateral pharyngeal wall (Fig 1). The mass surrounded the right common carotid artery and displaced the jugular vein laterally.
A, Contrast-enhanced axial CT scan shows lipomatous regions with thick septa (white arrow), a low-attenuation high-water-content component (black arrow), and sporadic heterogeneous high-attenuation calcified areas in the right common carotid space. The mass surrounds the right common carotid artery and displaces the jugular vein (black arrowhead) laterally. B, In this precontrast axial CT scan with a bone algorithm, the tumor ossification (black asterisks) is evident. C, A multiplanar reformatted image shows the mass with well-defined margins between the sternocleidomastoideus muscle and the lateral pharyngeal wall, displacing the right jugular vein. Thick septa (white arrows) and fat (white asterisks) are noted.
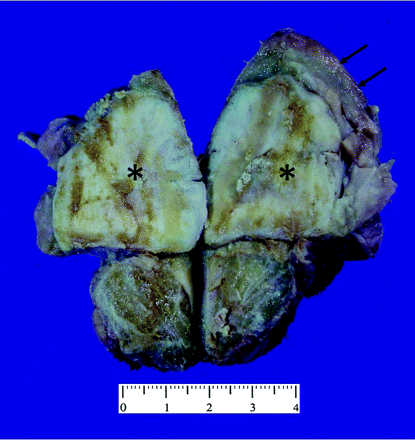
Photograph of the gross specimen shows the intramuscular sclerotic bone-forming lesion (black asterisks) with sharply delineated lipomatous caps (black arrows).
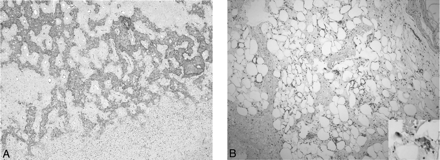
A, Photomicrographs of a biopsy specimen section obtained from the gritty nodular portion of the mass. Mineralized immature tumor bone trabeculae are present, and spindle-shaped tumor cells are seen adjacent to the tumor bone. The overall findings are consistent with osteosarcoma (HE, original magnification ×40). B, Photomicrographs of the lipoid portion. Mature-appearing adipose tissue combined with scattered lipoblasts (insert) (HE, original magnification ×100; insert, original magnification ×400).
The patient underwent a tumor resection via cervical approach. On gross inspection, a portion of the resected mass, measuring 5 × 5 × 4 cm, was gritty and nodular, with variegated tan-yellow to red-brown cut surfaces; another portion was lipoid tissue with approximately 8 cm of thickness, well-demarcated from the gritty nodular portion within the mass. Neither hemorrhage nor necrosis was identified. Microscopically, the nodular and gritty portion was composed of proliferating spindle-shaped cells that directly produced osteoid or immature tumor bone trabeculae. The lipoid area was characterized by mature-appearing adipose tissue, with only scattered lipoblasts. A diagnosis of a WDL juxtaposed to a high-grade osteosarcoma (ie, the DDL) was made. Five months after the initial surgery, the patient is disease-free without any signs of local recurrence or distant metastases.
Discussion
DDL occurs most frequently in patients in their seventh decade. Men and women are affected approximately equally.10 The areas of dedifferentiation are usually larger than 3 cm at imaging.10 The Armed Forces Institute of Pathology reported that 8.9% of all liposarcomas are dedifferentiated lesions, with 28.5% in the extremities, 66% in the retroperitoneum, and 6% in other locations.11
To the best of our knowledge, this is the ninth case of a DDL with osteosarcoma and the first case located in the neck reported in the literature.2–9
Radiographically, CT appearances of lipomatous tumors are based on the distribution of the fat and nonfat components, and patterns are classified into 3 types12: Type A tumors, homogeneous fatty tumors, are low-grade lipomatous neoplasms, including lipoma and lipomalike liposarcoma. Type B tumors, fat-attenuation tumors intermingled with higher attenuation components, are higher grade liposarcomas, including myxoid and mixed liposarcoma with pleomorphic or small cell types. Type C tumors, complex tumors containing both a fat component and a well-defined nonfatty component, are the typical pattern of DDL.12
In our case, osteosarcomatous components were approximately 5 cm. Calcification or ossification is a distinct imaging pattern of DDL. Osteosarcomatous and chondrosarcomatous dedifferentiation is the cause of such mineralization.
In the neck, the differential diagnosis for DDL with osteosarcomatous components and teratoma may be troublesome radiographically. CT of a teratoma demonstrates heterogeneous solid, fatty tissue, and cystic mass with specks of calcification.13 Other head and neck tumors that have fatty tissue include lipomas and lipoblastomas.
The clinical behavior of DDL, which reflects the high-grade histologic characteristics of most of these lesions, is more aggressive than well-differentiated liposarcomas. DDLs are treated with wide surgical excision and frequently with radiation therapy; chemotherapy may also be used as an adjunct. The disease is characterized by a tendency to recur locally in at least 40% of cases.1,14–19 Distant metastases are observed in 15%–20% of cases, with an overall mortality rate of 28%–30% at 5-year follow-up.3,20,21
Conclusions
We describe a case of DDL with an osteosarcomatous component in the neck. Radiologists should be familiar with the different location, and this case emphasizes the wide spectrum of DDL.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- Received September 22, 2010.
- Accepted after revision October 20, 2010.
- © 2012 by American Journal of Neuroradiology