Abstract
SUMMARY: A man with superficial siderosis showed improvement in symptoms and reduction in hemosiderin by MR imaging following treatment with deferiprone, a lipid-soluble iron chelator.
Superficial siderosis is a rare progressive neurodegenerative disease caused by subarachnoid hemorrhage leading to hemosiderin deposition on the pial surfaces of the central nervous system.1 While the exact mechanism is unknown, the amount and location of hemosiderin deposition on MR imaging correlate with symptoms and disease burden, with most patients presenting with a combination of hearing loss, cerebellar ataxia, and/or myelopathy due to involvement of the vestibular nerve (eighth cranial nerve), cerebellum, and spinal cord.2
Treatment for superficial siderosis is difficult because even after ablating the source of subarachnoid bleeding, the disease will likely progress because the hemosiderin deposition already present by the time of presentation overwhelms the patient's ability to clear it. Previous attempts to chelate the hemosiderin iron deposits have been limited to drugs that cannot effectively cross the blood-brain barrier. While the pathogenesis of superficial siderosis is likely due to iron overload, there has never been evidence that removing the iron would stabilize or reverse the disease process. Deferiprone (L1) is a lipid-permeable iron chelator that has been used for treatment of thalassemias and other iron metabolism disorders since 1989.3,4 We report on a patient with superficial siderosis who was treated with deferiprone for 1.5 years. We found a remarkable reduction in hemosiderin on MR imaging of the brain, associated with clinical improvement in symptoms. The patient information, MR imaging description, and discussion of a clinical trial of deferiprone in superficial siderosis follow.
Case Report
Our patient, a 65-year-old man, presented with headaches and seizures in 2006 and was found to have subarachnoid hemorrhage associated with a transverse sinus thrombosis by angiography. He was started on oral anticoagulants, which were discontinued 1 year later when he re-presented with ataxia, light-headedness, and reduced hearing. MR imaging confirmed the diagnosis of superficial siderosis. A work-up did not reveal the source of the bleeding.
The presumed mechanism of disease in superficial siderosis is toxicity from iron breakdown products causing glial cell death and eventual neurodegeneration. Previous attempts to use an iron chelator to treat superficial siderosis have failed because available chelators do not cross the blood-brain barrier. We proposed a trial of deferiprone, a lipid-soluble iron chelator, which has been shown to easily cross the blood-brain barrier and chelate iron in the central nervous system of animals.4 A trial dose of deferiprone at 30 mg/kg/day was started in January 2008 and was followed with weekly blood counts and monthly assessment of ferritin levels. Due to perceived adverse effects of joint and muscle aches and a low ferritin level, the dose was decreased to 15 mg/kg/day in July 2008. The patient reported resolution of his headaches, ataxia, and lightheadedness and stabilization of his hearing within 6–12 months.
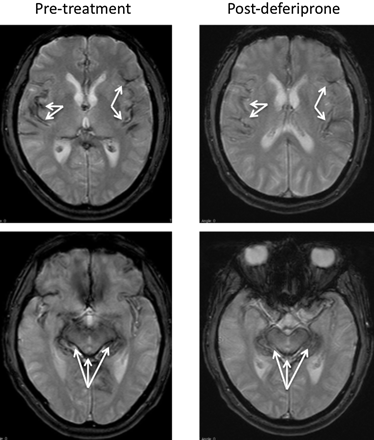
In July 2009, repeat MR imaging showed a reduction in hemosiderin deposition in the cortex and cerebellum compared with the MR imaging obtained in 2007, as shown in Fig 1. The decrease in hemosiderin deposition in these areas of the brain correlated with the improvement in his symptoms, specifically in the brain stem.
Axial T2* (TR/TE, 800/26 ms) brain MR images obtained before deferiprone therapy, compared with similarly positioned axial T2* (TR/TE, 560/26 ms) images obtained after deferiprone therapy, show the hypointensities along the pial surfaces representing hemosiderin deposition in the pretreatment cortex, brain stem, and cerebellar vermis. Postdeferiprone, the degree of hypointensity in those same brain areas is decreased; this change indicates reduced hemosiderin deposition. Arrows indicate hypointensity of the iron deposition.
Results of laboratory studies to monitor liver enzymes, complete blood counts, and ferritin that were drawn over the course of this period were stable. There was no evidence of liver toxicity or decrease in neutrophil counts; to our knowledge, these are rarely reported in the literature. The ferritin level initially dropped and then remained in the low-normal range throughout the course of his treatment. He currently remains on deferiprone. The decision to stop therapy will be based on complete resolution of MR imaging evidence of superficial siderosis.
Discussion
This is the first report of an effective therapy for superficial siderosis. A previous study on the natural history of this condition confirmed that the progressive decline in neurologic function with time is associated with an increase in hemosiderin-coated surface area in the nervous system.1 On the basis of animal studies of chronic subarachnoid hemorrhaging, the accumulation of iron and iron breakdown products is toxic to glial cells in the brain and spinal cord, particularly the Bergmann glia, which upregulate ferritin production to buffer the iron load.5 With time, the glia are overwhelmed by the iron products, and neurodegeneration ensues. Although there is 1 case report of reversal of disease progression with cessation of bleeding,6 most cases continue to progress, despite locating the source and stopping the bleeding.1
Previous attempts at treatment using available iron chelators have failed primarily because they do not cross the blood-brain barrier. Deferiprone is unique among iron chelators in that it crosses the blood-brain barrier to chelate iron in the central nervous system.4 This case report provides proof of concept because the MR imaging confirms a reduction in hemosiderin deposition in the brain. Most important, the reduction in hemosiderin deposition correlates with neurologic improvement, suggesting that superficial siderosis is a reversible condition once the iron products are removed from the nervous system.
On the basis of the improvement we have seen in this case, the US Food and Drug Administration approved an unblinded open-label trial of deferiprone in 10 patients with superficial siderosis in the United States. The trial has completed enrollment and is currently ongoing.
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- Received August 16, 2010.
- Accepted after revision August 18, 2010.
- Copyright © American Society of Neuroradiology