Abstract
BACKGROUND AND PURPOSE: There is no report regarding patency of elastase-induced aneurysms for more than a 2-year period. Our aim was to report aneurysm patency rates up to 5 years in the elastase-induced aneurysm model in rabbits.
MATERIALS AND METHODS: Twenty-five elastase-induced aneurysms were created in New Zealand white rabbits and followed for up to 5 years. Thirteen (52%) rabbits died during follow-up for reasons unrelated to the aneurysms. DSA was performed at 1 month and at 2 and 5 years in the 12 surviving subjects. Aneurysm patency and dimensions, including neck diameter and aneurysm width and height, were evaluated at each time point in relation to external sizing devices. Differences of aneurysm sizes (neck width and aneurysm width and height) among time points were compared by using the Student t test.
RESULTS: Eleven (92%) of the 12 aneurysms in the subjects that survived for 5 years remained fully patent throughout follow-up. A single narrow-neck aneurysm showed partial thrombosis at the 2- and 5-year time points.
CONCLUSIONS: Experimental elastase-induced aneurysms in rabbits demonstrate high rates of patency up to 5 years following creation. When planning for very long-term studies, investigators should plan for relatively high rates of mortality unrelated to aneurysm pathology.
Abbreviations
- AP
- anteroposterior
- DSA
- digital subtraction angiography
- IADSA
- intra-arterial DSA
- IVDSA
- intravenous DSA
- RCCA
- right common carotid artery
Long-term patency of experimental aneurysms is important in testing aneurysm-occlusion devices. Aneurysm models that have spontaneous thrombosis are considered of limited value in the evaluation of new endovascular occlusion devices. Notwithstanding the wide variety of experimental aneurysms described in numerous species, reports regarding long-term patency rates remain uncommon.1–4 Patency is poor in side-wall aneurysms in swine.1 Sidewall canine aneurysms appear to offer better patency rates than those of swine.2,3
The rabbit elastase-induced aneurysm model has been widely used for testing endovascular devices.5–20 Previous reports have noted excellent patency of untreated elastase-induced aneurysms ≤2 years after creation.18 In this study, we report the patency rate of elastase-induced aneurysms in rabbits at 5 years.
Materials and Methods
Aneurysm Creation
Elastase-induced saccular aneurysms were created in 25 New Zealand white rabbits (body weight, 3–4 kg) by using the rabbit elastase model. Some of these same subjects were reported in previous articles detailing the use of IVDSA19 and the patency rate of aneurysms up to 2 years.18 Our Institutional Animal Care and Use Committee approved all procedures. Detailed procedures for aneurysm creation have been described.5 Briefly, anesthesia was induced with an intramuscular injection of ketamine, xylazine, and acepromazine (75 mg/kg, 5 mg/kg, and 1 mg/kg, respectively). Using a sterile technique, we exposed and ligated the RCCA distally. A 1- to 2-mm bevelled arteriotomy was made and a 5F vascular sheath (Cordis Endovascular, Miami Lakes, Florida) was advanced retrogradely in the RCCA to a point approximately 3 cm cephalad to the origin of RCCA. Fluoroscopy (Advantx; GE Healthcare, Milwaukee, Wisconsin) was performed by injection of contrast through the sheath retrogradely in the RCCA, to identify the junction between the RCCA and the subclavian and brachiocephalic arteries. A 3F Fogarty balloon (Baxter Healthcare, Irvine, California) was advanced through the sheath to the level of the origin of the RCCA with fluoroscopic guidance and was inflated with iodinated contrast material. Porcine elastase (5.23 μ/mgP, 40.1 mgP/mL, approximately 200 U/mL; Worthington Biochemical, Lakewood, New Jersey) was incubated within the lumen of the common carotid artery above the inflated balloon for 20 minutes, after which the catheter, balloon, and sheath were removed and the RCCA was ligated below the sheath entry site.
DSA Follow-Up
Follow-up angiography was performed at each time point (1 month, 2 years, and 5 years after creation). Twelve (48%) of 25 rabbits survived for the full duration of the study, in which follow-up angiographic images at each time point were available in 11 aneurysms; the other aneurysm only had images at 2- and 5-year time points. Thirteen rabbits (52%) died from causes unrelated to the aneurysm itself: 5 from stroke, 2 from cancer, 4 from chronic liver failure, and 2 from anesthesia-related complications before 5 years after aneurysm creation. For the 1-month and 5-year time points, IADSA was performed, and it was also performed at 2 years. Details regarding the technique of IADSA and IVDSA have been previously reported.19 Aneurysm dimensions, including neck width and dome width and height, were measured and calculated from these DSA images in reference to external radiopaque sizing markers. DSA images also were used to evaluate interval changes in aneurysm dimension with time.
Statistical Analysis
Differences of aneurysm sizes (neck width and aneurysm width and height) among time points were compared by using the Student t test.
Results
Eleven (11/12, 92%) aneurysms remained completely patent angiographically throughout the 5 years following creation (Figs 1 and 2). At 2 years, a single narrow-neck aneurysm (neck size, 1.4 mm; width, 4:5 mm; ratio of width/neck, 3:2) showed partial thrombosis (Fig 3), which remained stable at 5 years.
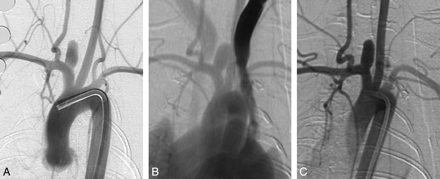
Serial DSA in an elastase-induced aneurysm. A, AP IADSA obtained 1 month after creation demonstrates an aneurysm cavity along the brachiocephalic artery, at the origin of the ligated RCCA. B, AP IVDSA in the same aneurysm 2 years after creation. C, AP IADSA in the same aneurysm 5 years after creation. Aneurysm dimensions remain constant throughout follow-up.
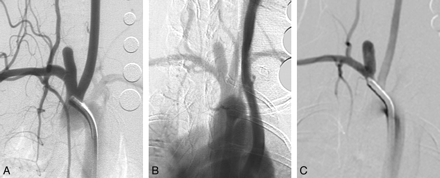
Serial DSA in another elastase-induced aneurysm. A, AP IADSA obtained 1 month after creation demonstrates an aneurysm cavity along the brachiocephalic artery, at the origin of the ligated RCCA. B, AP IVDSA in the same aneurysm 2 years after creation. C, AP IADSA in the same aneurysm 5 years after creation. Aneurysm dimensions remain stable throughout follow-up.
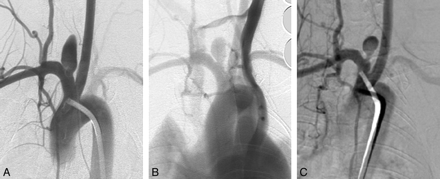
Serial DSA in an elastase-induced aneurysm undergoing partial thrombosis. A, AP IADSA 1 month after creation shows a patent aneurysm cavity. B, AP IVDSA at 2 years demonstrates partial thrombosis. C, AP IADSA 5 years after creation demonstrates similar partial patency compared with B.
Aneurysm Neck Dimensions
Mean neck widths at 1 month, 2 years, and 5 years after creation were 3.2 ± 1.1 mm, 3.0 ± 0.9 mm, and 3.0 ± 1.0 mm, respectively. There were no significant differences observed among these neck widths (P = .79).
Aneurysm Width
Mean aneurysm widths at 1 month, 2 years, and 5 years after creation were 3.9 ± 1.2 mm, 3.9 ± 1.2 mm, and 4.1 ± 1.0 mm, respectively. There were no significant differences among these aneurysm widths (P = .64).
Aneurysm Height
Mean aneurysm heights at 1 month and 2 and 5 years after creation were 8.9 ± 2.4 mm, 8.4 ± 2.3 mm, and 8.4 ± 2.1 mm, respectively. There were no significant differences among these aneurysm heights (P = .60).
Discussion
This study confirmed excellent long-term patency of the elastase-induced aneurysm models in rabbits. In this relatively small study of long-term patency, only a single narrow-neck aneurysm showed partial spontaneous thrombosis with time. Untreated human aneurysms also showed low rates of spontaneous thrombosis over time.21–24 Indeed, observation of spontaneous aneurysm thrombosis in humans is the subject of case reports.22–24 As such, our observed long-term patency rate of the elastase-induced aneurysms offers further support for the application of this aneurysm model, not only for evaluation of endovascular devices but also for aneurysm hemodynamics and biology.25,26
The short- and long-term patency of the elastase-induced aneurysm model has previously been reported. The model demonstrates early growth, up to 3 weeks following creation, after which aneurysm dimensions stabilize.17 This early growth may reflect either ongoing injury to the elastic lamina or ongoing interplay between aneurysm hemodynamics and mechanical integrity. Previous studies have demonstrated ongoing patency of this model for up to 2 years.18 The current study confirms ongoing patency in untreated aneurysms up to 5 years after creation.
Long-term patency rates of most types of experimental aneurysm models are rarely reported. One study reported the patency of 326 vein patch aneurysms in 310 canines during a 6-year period. Of these, 102 were sidewall (lateral) and 224 were bifurcation aneurysms. Spontaneous occlusion occurred in 9 (9/102, 9%) of the sidewall aneurysms and in only 1 (1/224, 0.4%) of the bifurcation aneurysms.2 Future model development would ideally offer such long-term patency reports.
This study had several limitations. The cohort was small, limited by the substantial financial requirements for maintaining experimental subjects for up to 5 years. Thus, the impact of aneurysm neck size or aneurysm dimension could not be determined. Second, a relatively high proportion of the rabbits in our study died from age-related pathologies, including chronic liver failure. One might argue that if we based our patency rate on the initial cohort instead of the surviving subset, our actual patency rate would have been 11 (44%) of 25 subjects. In any event, the high age-related mortality rate should be carefully considered by investigators planning very long-term rabbit aneurysm studies, with extra subjects added at the outset as needed.
Conclusions
Experimental elastase-induced aneurysms in rabbits demonstrate high rates of patency up to 5 years following creation. When planning for very long-term studies, investigators should plan for relatively high rates of mortality unrelated to aneurysm pathology.
Footnotes
-
Supported by the National Institutes of Health grant R01 NS46246.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.
- 2.
- 3.
- 4.
- 5.
- 6.
- 7.
- 8.
- 9.
- 10.
- 11.
- 12.
- 13.
- 14.
- 15.
- 16.
- 17.
- 18.
- 19.
- 20.
- 21.
- 22.
- 23.
- 24.
- 25.
- 26.
- Received November 17, 2009.
- Accepted after revision January 9, 2009.
- Copyright © American Society of Neuroradiology