Abstract
BACKGROUND AND PURPOSE: Fluid-attenuated inversion recovery (FLAIR) vascular hyperintensities (FVH) are commonly encountered on MR imaging studies performed shortly after the onset of acute ischemic stroke. Prior reports have speculated regarding the pathogenesis of this finding, yet definitive correlative angiographic studies have not been performed. We studied the pathophysiologic and hemodynamic correlates of FVH on conventional angiography and concurrent MR imaging sequences.
MATERIALS AND METHODS: Retrospective review of FLAIR and gradient-refocused echo MR imaging sequences acquired immediately before conventional angiography for acute stroke was conducted in a blinded fashion. The presence, location, and morphology of FVH were noted and correlated with markers of thrombotic occlusion and collateral flow on angiography. Angiographic collaterals were graded on a 5-point scale incorporating extent and hemodynamic aspects.
RESULTS: A prospective ischemic stroke registry of 632 patients was searched to identify 74 patients (mean age, 63.4 ± 20 years; 48% women) having undergone FLAIR sequences immediately before angiography. Median time from FLAIR to angiography was 2.9 hours (interquartile range, 1.1–4.7 hours). FVH were present in 53/74 (72%) of all acute stroke cases with subsequent angiography. FVH distal to an arterial occlusion were associated with a high grade of leptomeningeal collateral blood flow.
CONCLUSIONS: FVH are observed in areas of blood flow proximal and distal to stenosis or occlusion and are noted with more extensive collateral circulation.
Advances in MR imaging allow better characterization of tissue and vessel status in the setting of acute stroke. One frequently encountered imaging finding in acute ischemic stroke is high signal intensity on fluid-attenuated inversion-recovery (FLAIR) MR imaging sequences within blood vessels (Fig 1). The etiology of these FLAIR vascular hyperintensities (FVH) is unknown, though some have postulated that they may be due to slow flow1,2 or clot within blood vessels.3 FVH can be found in the setting of acute stroke due to large-vessel stenosis4 or occlusion.3 The clinical significance of FVH remains obscure, and this relatively subtle imaging finding is often transient.3
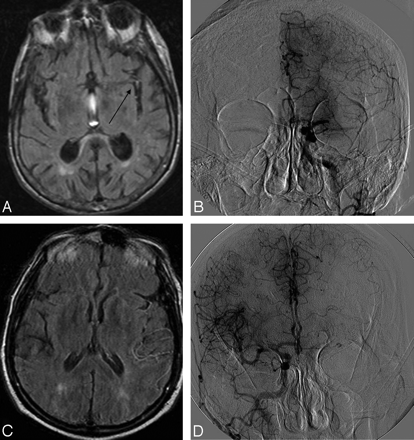
Two cases of retrograde leptomeningeal collateral flow in areas corresponding to FVH. A and B, Case 1: FLAIR demonstrates FVH (arrow) in the Sylvian fissure with an angiogram (B) showing grade 3 collaterals from the ipsilateral ACA. C and D, Case 2: FLAIR (C) demonstrates temporoparietal FVH with ACA-MCA leptomeningeal collaterals on the angiogram (D).
Complementary methods of imaging may help to understand the underlying etiology of FVH. We sought to characterize FVH better by comparing findings on digital subtraction angiography (DSA) performed within 6 hours of MR imaging in the setting of acute ischemic stroke. Particular emphasis was placed on evaluating blood flow in and around hyperintense vessels.
Materials and Methods
Data on 632 consecutive patients with acute ischemic stroke evaluated by the stroke team at a single university hospital were retrospectively analyzed. Subjects were included in this study if they presented with acute ischemic stroke or transient ischemic attack (TIA) and underwent MR imaging of the brain followed by cerebral DSA within 6 hours of the MR imaging. Patients were excluded if they presented with aneurysm or hemorrhagic stroke, if their MR imaging did not include a FLAIR sequence, and if their MR imaging or DSA images were not available for review. All patients underwent standard DSA for consideration of endovascular recanalization.
Additional data obtained included demographic information, timing of the initial MR imaging, angiograms, and follow-up MR imaging when available. In addition to FLAIR sequences, diffusion-weighted images (DWI), perfusion-weighed images (PWI), T2*-weighted gradient echo, and 3D time-of-flight intracranial MR angiography (MRA) sequences were reviewed if available to confirm the vascular territory of the ischemia.
Two investigators reviewed the relevant studies. The first reviewed only the MR images, whereas the second reviewed only the DSA images. Each reader was blinded to the reading of the other.
The MR imaging reader evaluated the FLAIR, T2*-gradient-refocused echo, DWI, PWI, and MRA sequences performed within 6 hours before DSA. Any available follow-up MR imaging studies were also reviewed. The quality of MR imaging was characterized as adequate or inadequate. The presence or absence of FVH was noted by using prespecified criteria.1,5,6 Criteria for FVH were focal, tubular, or serpentine hyperintensity relative to gray matter in the subarachnoid space or extending into the brain parenchyma. FVH were coded as within or outside the arterial territory of DWI abnormality. Location of the FVH relative to the DWI abnormality was also noted. Anatomic location of FVH and the presence of branching were detailed. Follow-up MR images were reviewed for the presence of FVH, increased parenchymal signal intensity of FLAIR consistent with infarction, and relation of infarction to FVH.
Angiographic images obtained ≤6 hours after MR imaging were reviewed. The location of occlusions and stenoses within arterial segments was noted and graded. The direction of flow in blood vessel segments and routes of collateral blood flow were detailed. Angiographic collaterals were graded on a 5-point scale (Table 1), and all data were abstracted by using a standardized form.7 The study was approved by the University of California, Los Angeles institutional review board.
Characteristics and imaging findings in study patients
The ASITN/SIR collateral-flow grading system to determine angiographic collateral grade on pretreatment angiography
Results
Seventy-four patients fulfilled inclusion criteria (Table 1). Mean age was 63.4 years, and 48% were women. All patients underwent urgent investigation with MR imaging, which included FLAIR sequences within 6 hours before conventional angiography. The median time from MR imaging to angiography was 2.9 hours (interquartile range [IQR], 1.1–4.7 hours). FVH were noted in 53/74 (72%) studied patients presenting with acute stroke seen urgently for endovascular recanalization.
When present, FVH were found within the vascular territory of the ischemic stroke in all studied patients as determined by initial DWI, MR imaging, and angiography. FVH were present in 33/35 (94%) cases of proximal middle cerebral artery (MCA, M1) ischemia (occlusion/stenosis) and in only 2/5 cases of vertebrobasilar ischemia. FVH were seen with cases of internal carotid artery (ICA) occlusions extending into the MCA (carotid L) and extending into both the anterior cerebral artery (ACA) and the MCA (carotid T). FVH were also seen with combined ICA and MCA occlusion. Across all cases, FVH were never visualized outside the involved vascular territory.
FVH were most commonly situated in the Sylvian fissure (94%) or adjacent to or within the temporal (89%), frontal (53%), and parietal lobes (34%). Discontinuity or a gap in FVH was defined as a segment of artery with decreased signal intensity on FLAIR relative to both proximal and distal vessels. The presence of an FVH gap was noted in 26/53 (49%) cases with FVH.
In a subset of 29 patients with angiographically documented isolated MCA occlusion, all except 1 had evidence of FVH distal to the occlusion on preprocedural MR images. In these 28 cases of MCA occlusion with demonstrated FVH, there was a high grade of leptomeningeal collateral blood flow appreciated. Twenty-two cases (79%) demonstrated excellent retrograde leptomeningeal collateralization of grade 3 or 4 (Fig 2). Six cases demonstrated some leptomeningeal collaterals (grades, 1 and 2). There were no cases with FVH noted distal to the MCA occlusion and absence of leptomeningeal collaterals (grade, zero). The lone case of isolated MCA occlusion with absence of FVH had a collateral grade of zero.
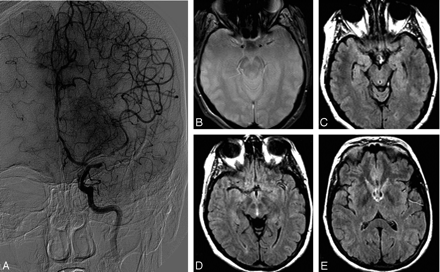
A case showing grade 4 collaterals on angiography (A) and FVH (C−E) distal to thrombus, demonstrated as an area of blooming artifact (B).
Of the 74 patients included in this study, 44 had follow-up MR imaging within 72 hours of the initial MR imaging (median, 8.2 hours; IQR, 6.0–19.6). FVH were noted on only 18 of 44 follow-up MR images. In all patients with FVH on follow-up MR images, there was evidence of FVH on the preangiographic scan. In the 18 patients with persistent FVH, 16 had concurrent interpretable MRAs. All 16 of these patients showed absence or severe reduction of flow signal intensity in the affected arterial territory. There were 15 cases of follow-up imaging in which an MRA was performed and demonstrated normal flow signal intensity in the affected arterial territory; there was no evidence of persistent FVH in any of these cases.
Discussion
This study describes the angiographic characteristics of patients who underwent FLAIR imaging and DSA within 6 hours in the setting of acute stroke, who were being considered for revascularization. MR imaging is increasingly used to diagnose acute ischemic stroke,8 and recent studies have demonstrated that MR imaging may be superior to CT in the initial evaluation.9 Because time is of the essence in acute stroke, MR imaging sequence protocols for acute stroke need to address the impact and time of acquisition. FLAIR sequences have become a routine part of many standard MR imaging protocols but have been primarily used for imaging brain parenchyma10 and the subarachnoid space.11 The role of vessel imaging with FLAIR in the setting of acute ischemic stroke is a topic of ongoing investigation, and FLAIR is not currently used in the routine assessment or management of patients with acute stroke.1,12 The underuse of FLAIR vascular imaging may be due to the relative subtlety of this finding and the unclear implications in acute ischemic stroke.
This study of FVH with subsequent angiography better elucidates the basis of FVH, a frequently encountered clue to the pathophysiology of cerebral ischemia. Our analyses demonstrate that FVH are often noted in the setting of impaired hemodynamics and retrograde collateral blood flow. FVH were never seen in the hemisphere contralateral to cerebral ischemia and were noted both proximal and distal to intracranial occlusive lesions. The most frequent site of FVH was adjacent to the insular region, in the setting of anterior circulation occlusions with good collateral circulation. FVH were seen in parts of the vascular territory affected, whereas slow flow may be expected along most of the vascular territory. There may be a threshold below or above which FVH are not noted.
Hyperintensity of vessels on FLAIR was first described by Kamran et al1 and was seen in 30 of all 304 (10%) MR images of patients with stroke, but in 30 of 66 (45%) scans were obtained within 24 hours of symptoms, indicating that this finding may be transient. All patients with vessel hyperintensities had large-vessel occlusion or high-grade stenosis on MRA. Only 8 patients in this study had concomitant cerebral angiography, which demonstrated retrograde leptomeningeal collateral flow in all cases.
Studies evaluating the presence of FVH in acute stroke settings have shown a positive correlation with brain infarction within the same territory on follow-up imaging.12 Of patients with FVH on hyperacute MR imaging, 85% went on to have infarction within the same territory. Other studies have confirmed that FVH are rarely seen in patients who do not have high-grade stenosis or occlusion of cerebral vessels.4 These findings support the belief that FVH are found in the setting of acute large-vessel occlusion, a condition highly correlated with subsequent stroke.
Although there is some disagreement about the underlying basis of FVH, most authors agree that some form of hemodynamic alteration is involved. The finding of FVH is unique to stroke and has not been reported in other conditions. It has been studied in intracranial stenosis4 and hyperacute ischemic stroke.1,3,12 Correlative studies have been performed with conventional angiography, single-photon emission CT,1 postcontrast T1 MR imaging, PWI,5 and MRA.12 These studies consistently demonstrate hemodynamic alterations of blood flow with FVH. The presence of FVH may indicate persistent large-vessel occlusion and thus a higher risk of stroke in the setting of TIA.13
The imaging signal-intensity characteristics of FVH are due to the sluggish flow of blood proximal and distal to large arterial occlusion. This altered flow results in the loss of flow voids on FLAIR because vessels appear hyperintense against a dark CSF background. In the setting of normal hemodynamics, there is loss of signal intensity in the blood vessels because blood protons have moved out of the imaging section when the section-selective 180° pulse is applied, and a spin-echo is not formed. The longer the TE of a pulse sequence (as seen in T2, proton-weighted, and FLAIR sequences), the more evident are the flow voids. Absence of flow voids on proton-attenuation MR imaging sequences has previously been correlated with FVH.2
We found that FVH were associated with a high grade of collateral circulation, most often grade 3 or 4 and never zero. This finding is consistent with the observation that FVH reflect altered hemodynamics. In the setting of proximal arterial occlusion and robust collateralization, brain parenchyma distal to the occlusion is nourished via retrograde leptomeningeal collaterals.14 Although anterograde blood flow is relatively fast, retrograde flow is more sluggish. On angiography, retrograde blood flow arrives later than anterograde flow, often during the angiographic venous phase. This difference contrasts with the absence of flow, in which FVH may not be evident. The persistence of FVH on follow-up MR imaging was associated with decreased or absent flow signal intensity on MRA, suggesting persistent vessel occlusion. In contrast, the absence of FVH was seen in the setting of return of MR imaging flow signal intensity and also in settings with completed infarction.
Limitations of this study include the narrow population: patients with acute stroke who underwent angiography for possible endovascular intervention. MR imaging and angiographic studies were reviewed by physicians blinded to the other imaging technique, and there could be inter-reader variability in interpretation. This population is expected to have a very high rate of large-vessel occlusion and salvageable brain penumbra being considered for endovascular intervention and may not be generalizable. The strengths of this study include a close temporal relationship from initial MR imaging to angiography and high rates of follow-up neuroimaging.
The finding of FVH on MR imaging in acute stroke should suggest consideration of proximal arterial disease leading to retrograde collateralization. The finding is often transient and, when persistent, may reflect continued hemodynamic impairment. Prospective studies are warranted to evaluate the relative incidence of FVH systematically in patients presenting with acute stroke, and this neuroimaging marker may be especially important in instances in which MRA or perfusion MR imaging is unavailable or degraded by artifacts.
Footnotes
This work was supported by Grant K23NS054084 (DSL) from the National Institute of Neurologic Disorders and Stroke.
References
- Received May 6, 2008.
- Accepted after revision September 25, 2008.
- Copyright © American Society of Neuroradiology