Abstract
SUMMARY: We present a case series demonstrating abnormal regional cerebral hyperperfusion associated with migraine headache using arterial spin-labeling (ASL). In 3 of 11 patients, regional cortical hyperperfusion was demonstrated during a headache episode that corresponded to previous aura symptoms.
Migraine is a common condition affecting 12% of the United States population per year.1 Migraines classically present with unilateral debilitating, painful headaches associated with photophobia, phonophobia, nausea, and vomiting.2 Less than 30% of migraines are preceded by auras, or focal neurologic deficits. Most commonly, the neurologic deficits are visual, but sensory and motor deficits can also occur. The pathophysiology of a migraine is complex and is not completely understood. The vascular theory proposes that the migraine aura is associated with vasoconstriction and the subsequent headache is associated with vasodilation and hyperperfusion.3,4 The second theory is that a contiguous wave of neuronal depression spreads through the cortex causing the aura symptoms.5–10 The vascular changes have been evaluated with nuclear medicine studies including single-photon emission CT and positron-emission tomography as well as blood oxygen level–dependent functional MR imaging and T2* dynamic susceptibility contrast (DSC) MR perfusion imaging.3,8,9,11–13 These methods are limited in the evaluation of the patient with migraine because of the dynamic nature of the perfusion abnormalities.
Spin tag perfusion imaging is an MR imaging method that measures quantitative cerebral blood flow (CBF).14 The advantages of arterial spin-labeling (ASL) compared with conventional perfusion techniques include repeatability, absolute quantification, and the avoidance of intravenous contrast administration.15,16 We performed a retrospective analysis of MR imaging examinations including ASL perfusion in patients with an indication of headache from the past 12 months and identified 11 clinical patients with a history of migraine headache. We present the 3 patients with migraine-associated regional cerebral hyperperfusion corresponding to the clinical presentation.
Case Reports
Patient 1
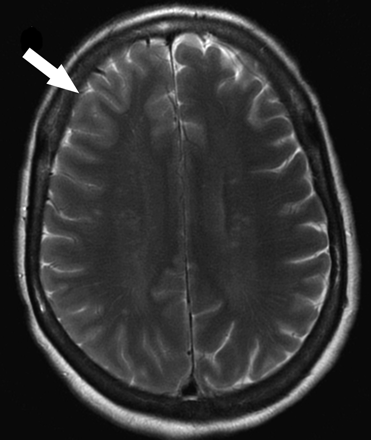
A 55-year-old woman with a history of hemiplegic migraines presented with new-onset right frontal headache, left-sided numbness, weakness, and dysarthria. The patient took prochlorperazine (Compazine) at home without relief of her symptoms. We performed cerebral MR imaging 6 hours after onset of symptoms using our standard stroke protocol including conventional sequences and ASL perfusion. Conventional MR imaging sequences demonstrated subtle sulcal effacement and mild edema in the right frontal cortex without evidence of diffusion abnormality (Fig 1).
Patient 1. Subtle sulcal effacement and mild cortical edema are seen in the right frontal lobe on the axial T2-weighted image (arrow). Diffusion-weighted sequences (not shown) were normal.
We generated quantitative CBF maps using quantitative imaging of perfusion and a single subtraction with thin-section inversion time (TI)1 periodic saturation with a flow-sensitive alternating inversion recovery sequence as described previously.17 This sequence generates 60 tag and control image pairs. Imaging parameters are as follows: TE, 28 ms; TI, 1800 ms, TI1s, 1200 ms; TI, 2000 ms; TR, 3000 ms; receiver bandwidth, 62.5 kHz; flip angle, 90°; FOV, 24 cm (frequency) × 18 cm (phase); acquisition matrix, 64 × 48 (11 sections, 8-mm thickness, 0-mm section gap); and frequency encoding direction anterior/posterior. We performed the sequence using 1.5T Signa Excite HD scanners (GE Healthcare, Waukesha, Wis). A diffusion gradient with an equivalent b-value of 5.25 mm2/s was added to suppress intra-arterial spins.18 We computed and inserted perfusion maps into the clinical PACS using a fully automated distributed processing pipeline.19 Briefly, the perfusion maps were segmented on the basis of the anatomic T1-weighted image and scaled by the mean signal intensity of blood allowing for voxel-wise computation of absolute CBF maps. We colorized the perfusion maps using a standard scale with a JPEG of the resulting image series automatically sent to the PACS along with the gray-scale perfusion data.
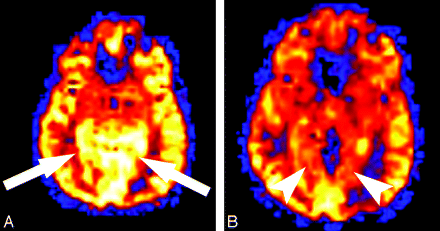
ASL images showed marked regional hyperperfusion in the right frontal and parietal cortex (Fig 2A). Repeat MR imaging 6 days later demonstrated resolution of the hyperperfusion on ASL (Fig 2B). After a complicated hospital course, the patient's hemiplegia and other neurologic symptoms completely resolved.
Patient 1. A, Quantitative CBF map generated from the ASL sequence shows significant hyperperfusion (arrow) within the cortex of the right cerebral hemisphere corresponding to the signal intensity abnormalities on the conventional MR imaging sequences. Region of interest placed over the frontal gray matter shows a mean CBF in the right hemisphere of 128.9 compared with the left frontal hemisphere mean CBF of 56.6 mL/100 g tissue/min. B, Quantitative CBF map obtained 6 days after the initial examination shows resolution of the hyperperfusion within the cortex of the right frontal and parietal lobes. Mean gray matter CBF in the right and left frontal lobe decreased to 57.7 and 61.6 mL/100 g tissue/min, respectively.
Patient 2
A 52-year-old woman with a history of transient ischemic attacks and migraine headaches presented with “shaky” vision episodes lasting 30 seconds. The patient later developed a severe migraine headache during her admission, which required intravenous administration of hydromorphone (Dilaudid). Results of conventional MR imaging and MR angiographic (MRA) sequences obtained 24 hours after visual symptom onset and during the headache were normal. Using the identical technique as in patient 1, ASL showed hyperperfusion in the medial occipital lobes bilaterally (Fig 3A). The headache and visual symptoms resolved during the next 24 hours, and the patient was discharged home and was prescribed topiramate (Topamax). Repeat MR imaging 6 months after her hospitalization showed resolution of the hyperperfusion (Fig 3B).
Patient 2. A, Quantitative ASL CBF map shows regional hyperperfusion (arrows) in the medial occipital lobes on a background of global hyperperfusion in this patient who underwent imaging examination during a migraine episode. Mean gray matter CBF measured 162.2 and 138.7 mL/100 g tissue/min, respectively, in the medial left and right occipital cortex. B, Quantitative CBF map obtained 6 months after the migraine episode shows resolution of the regional hyperperfusion in the medial occipital lobes (arrowheads). The underlying global hyperperfusion also resolved. Mean gray matter CBF decreased from 162.2 to 107.5 mL/100 g tissue/min in the medial left occipital cortex and from 138.7 to 112.9 mL/100 g tissue/min in the medial right occipital cortex.
Patient 3
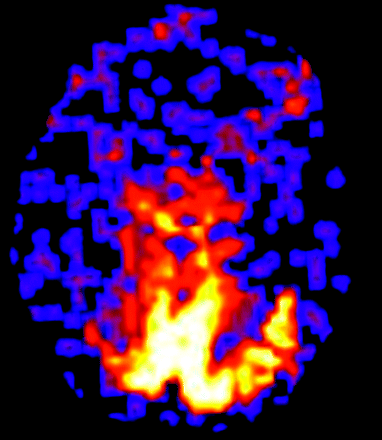
An 87-year-old woman presented with recent onset of daily episodes of “lights” in her visual fields. Approximately 20% of the time, the vision change was accompanied by a sharp left-sided headache, nausea, and photophobia. From a clinical standpoint, she was felt to have migraine headaches with aura, but because of the late onset in life, there was concern for arteritis. Results of CT of the head and conventional MR imaging and MRA sequences 12 hours after onset of symptoms were normal. The patient treated her headache with acetaminophen (Tylenol). Results of ASL, with use of the same technique as in patient 1, demonstrated regional hyperperfusion in the medial occipital cortex in the setting of globally decreased perfusion (Fig 4). The global hypoperfusion was attributed to the marked age-advanced cerebral atrophy. The patient refused to start new prophylactic migraine therapy and wished to treat her headaches with acetaminophen. She continued to have weekly headaches and daily vision changes.
Patient 3. Quantitative CBF map shows marked regional hyperperfusion in the occipital lobes bilaterally. This 87-year-old patient had marked global cerebral atrophy accounting for the relative hypoperfusion of the remaining cortex.
Discussion
ASL perfusion MR imaging was first described more than 10 years ago20 and has been an active area of research.21–27 ASL has several advantages compared with conventional bolus-tracking methods for migraine imaging. ASL requires no gadolinium-based contrast, can be repeated in the same session, and provides information on absolute CBF. These advantages mean that ASL can track and quantify the evolving perfusion abnormalities associated with migraines. The repeatability may ultimately allow for near real-time monitoring of cerebral perfusion during abortive migraine therapy. This method has been attempted with contrast-based techniques but was unsuccessful because of the time interval required between imaging and abortive therapy.11,28
The vascular theory of migraines proposes that migraines are a dynamic form of a cerebral pathologic disorder with clinical features and perfusion abnormalities evolving with time.3 Patients who undergo imaging examination during an aura characteristically have hypoperfusion, whereas those who undergo imaging examination during the headache phase characteristically show hyperperfusion.4,29,30 The findings in this case series provide support for the vascular theory of migraine headaches. However, the more popular migraine theories suggest a contiguously spreading wave of cortical neuronal depression.12,31 The perfusion changes may not be the inciting event; rather, the perfusion abnormality may be the response of the autoregulatory mechanisms to underlying neuronal depression.5–10,12,13,29,31–33 This concept is supported by case reports of persistent hyperperfusion despite resolution of headache with abortive therapy.3,11
Although the exact cause of a migraine has yet to be completely defined, migraines are clinically classified on the basis of the presence or absence of aura phenomenon. In addition, the aura phase is characterized by the types of symptoms. Most commonly, the visual pathway is affected; however, patients can present with motor or sensory symptoms.2 When patients present with aura-associated motor and sensory loss, they are classified as hemiplegic migraines.2,34 Familial and sporadic forms exist, with genetic mutations having been identified.32 Diagnosis is usually made on clinical examination; however, the presentation may mimic entities such as seizure, meningitis, and stroke.32,35
Conventional imaging findings during hemiplegic migraine typically show cortical edema without infarction.3,11 DSC and nuclear medicine studies have shown postaura hyperperfusion in the cortex affected by the hemiplegic migraine.3,11,36–38 As opposed to ASL, nuclear medicine studies and contrast bolus techniques are not easily repeatable, require radiopharmaceuticals that must be prepared in advance, and can require multi-day examinations to avoid double dosing.
ASL does have limitations when migraine-associated hyperperfusion is being evaluated. Additional causes such as neoplasms, seizure foci, and infarctions with luxury perfusion frequently show regional hyperperfusion and may be associated with headache.14,39 ASL perfusion imaging must be interpreted within the clinical context and in conjunction with conventional imaging. ASL does provide quantitative CBF, but it currently does not provide information regarding the mean transit time or cerebral blood volume.15,18 Thus, although blood flow may be increased in our migraine cases, we do not know if the hyperperfusion is because of increased cerebral blood volume, faster transit times, or a combination of the 2. Other methods such as CT perfusion and DSC perfusion imaging provide all 3 parameters, which may elucidate the pathophysiologic mechanisms responsible for the hyperperfusion.11 Although both techniques can provide whole brain coverage, they also both suffer from susceptibility artifacts, which can limit evaluation of the skull base and temporal lobes.
ASL sequences have been studied regarding ischemic states and blood flow quantification of brain tumors, but limited research exists on regional hyperperfusion states.14,27,39,40 The 3 patients presented here demonstrate the capability of ASL to reveal regions of hyperperfusion in the patient with symptomatic migraines. The 8 of 11 patients with a history of migraine, but who were asymptomatic during imaging, showed no perfusion abnormalities. ASL perfusion analysis may help identify patients with vascular or hemiplegic migraines by showing associated hypoperfusion during the aura state and hyperperfusion during the headache state (Figs 2–4). We believe that the hyperperfusion seen in the symptomatic patient group supports the vascular theory in patients with migraine aura. However, the perfusion imaging features can mimic an infarction with reperfusion and should be correlated with the clinical history and conventional imaging. These patients demonstrate how the cerebral perfusion correlates with the clinical symptoms as well as with the dynamic nature of patterns of migraine perfusion.
Conclusion
Migraine associated perfusion anomalies has been described, but until now, a safe, nonradioactive, nonintravenous contrast, easily repeatable imaging study has not been available. When patients with migraine symptoms and neurologic deficits are undergoing imaging examination acutely, conventional imaging sequences may be normal. However, regional hyperperfusion may be seen in the affected cerebral cortex corresponding to the neurologic manifestations during the aura. The perfusion imaging should be interpreted in conjunction with conventional sequences because other causes may have similar clinical histories and perfusion findings. As ASL becomes more widely implemented, its unique advantages compared with conventional perfusion studies give this perfusion sequence significant potential in the evaluation of the patient with acutely symptomatic migraines.
Footnotes
There are no conflicts of interest. Research support was provided by the National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, and the Human Brain Project through grants EB004673 and EB004673-02S2. This case has not been otherwise published.
References
- Received November 30, 2007.
- Accepted after revision February 7, 2008.
- Copyright © American Society of Neuroradiology