Abstract
BACKGROUND AND PURPOSE: Current models of brain function propose that number processing involves the interaction of different neuronal networks. Our purpose was to use functional MR (fMR) imaging to elucidate the brain regions engaged by multiplication.
METHODS: Eighteen adults underwent fMR imaging while performing matching, multiplication, and control tasks. For each task, three or four single-digit or low-value double-digit numbers were presented serially followed by a 12-second delay. A target stimulus then appeared and subjects made a judgement by pressing a button box that recorded responses. During the matching task, subjects judged whether the target stimulus matched one of the previous numbers. During the multiplication task, subjects judged whether the target stimulus was the product of the previous numbers. For the control task, the numbers were always zeros, and the subjects responded to a target stimulus that was always four zeros. Composite statistical parametric maps of the time course of activation comparing the control task with the matching and multiplication tasks, respectively, were generated and the significance of signal changes was estimated by randomization of statistical parametric maps.
RESULTS: The matching and multiplication tasks resulted in activation (P < .005) in the medial superior frontal gyrus; the anterior cingulate gyrus; the intraparietal sulci, bilaterally; the right superior frontal sulcus bilaterally; the middle, inferior and precentral frontal gyri (left greater than right); the left basal ganglia; and the right lateral and inferior occipital gyri. There was a larger area of early activation in the right middle frontal gyrus during the matching task compared with the multiplication task, and there was a longer interval of activation in the left middle frontal gyrus during the multiplication task (10 seconds) than in the matching task (6 seconds).
CONCLUSION: Multiplication and memory of numbers share an integrated network of brain regions. The left frontal lobe, an area also involved in memory and language processes, appears to play an important role in multiplication.
Mathematics is an intellectual process that involves a variety of cognitive functions, including visuospatial skills, memory, attention, and semantic representation (1, 2). A more elementary level of number processing—the ability to judge the numerosity of a stimulus, which is the property of a stimulus that is defined by the number of elements it contains that can be discriminated—can be seen not only in adult humans, but also in human infants and in some animal species (3–18).
To represent numerosity, current models propose that adults use both language-dependent and language-independent processes (19, 20). When number processing requires exact arithmetic knowledge, cognitively proficient adults have available a vocabulary of known answers organized in a semantic network that uses brain mechanisms involved in processing language. Temporary storage of information (working memory) may be needed for more complex problems. When adults represent number magnitude during tasks that require number approximation or quantity manipulation, a relatively language-independent process that depends on brain regions comprising visuospatial, nonverbal networks is believed to be engaged.
Imaging studies of cerebral activation during mathematical tasks have shown that several brain regions are involved in number processing. Using intracarotid 133XE-injection measurements of regional cerebral blood flow, while subjects serially calculated 503, demonstrated increased regional cerebral blood flow in the prefrontal, inferior frontal (including Broca's), and angular cortices bilaterally (21). Functional MR (fMR) imaging during a similar task revealed inferior parietal and lateral frontal activation (22). Positron emission tomography (PET) of nine subjects who covertly read mathematical problems without doing calculations and who performed calculations on visual stimuli showed that the prefrontal area is important in arithmetic operations and that the posterior superior temporal gyrus plays a role in both comprehension and computation (23). Another PET study of eight subjects, which used number comparison and multiplication tasks, reported that a large number of brain regions were active during multiplication tasks. These included the occipital lobe regions (left fusiform and lingual gyri and right cuneus), the precentral gyrus, the supplementary motor area, the left basal ganglia, and the inferior parietal lobules bilaterally (24). A recent study that measured reaction times, fMR imaging of activation sites, and event-related potentials (ERPs) support the concept that number processing has both language-dependent and language-independent components (25). Arithmetic tasks that required exact answers relied more on verbal representations of numbers, primarily in the left frontal lobe, and tasks that required estimation or approximation depended more on visuospatial networks centered on the intraparietal sulci bilaterally. Lesion and ERP studies support involvement of occipital gyri, parietal lobes, basal ganglia, and frontal lobes in number processing (1, 26–39).
In this study, we compared a matching task with a multiplication task. During the matching task, subjects viewed the serial presentation of three numbers and then judged by button press, after a 12-second interval, whether a target stimulus was one of the three numbers just presented. Subjects viewed numbers, remembered numbers, attended to the stimuli, but did not perform calculations. The multiplication task consisted of serial presentation of four single-digit or low-value double-digit numbers. Subjects multiplied the numbers mentally during a 12-second interval after which they judged by button press whether a target stimulus was the correct product of the four numbers just presented. This task required that subjects viewed numbers, remembered the numbers, attended to the task, and multiplied the numbers together. As a control, we presented a zero serially four times, and after a 12-second delay, asked subjects to respond by button press to four zeros presented together. During this task, subjects viewed numbers, attended to the task, but did not have to memorize a number or perform any calculations. We expected the matching and multiplication tasks to activate brain regions engaged by number visualization, comparison of number quantity, and memory. Compared with the matching task, which requires memory of numbers but no calculation, the multiplication task should reveal brain regions more involved in calculation, comparison of number quantity, and semantic representation of number facts.
Methods
Task
Eighteen healthy, right-handed subjects (nine men and nine women) participated in this study. All subjects had attended college. Mean subject age was 32 years. Math education and occupation of subjects are shown in the Table. Prior to entering the magnet, we provided the subject with a description of the task, a consent form to read, and a brief orientation to MR imaging. All subjects gave written informed consent. The institutional review boards at our respective institutions approved this study.
Stimuli were presented in random order across eight imaging runs, each run consisting of nine trials: three multiplication trials, three matching trials, and three control trials, counterbalanced across runs to control for position effects. All trials consisted of three or four stimuli presented for 500 milliseconds, with a delay of 300–400 milliseconds (the interstimulus interval was 800–900 milliseconds). Stimulus presentation was followed by a delay of 12 seconds, after which the target stimulus was presented for 1000 milliseconds. The total trial interval was 16.5 seconds. The words “Match,” “Multiply,” or “Rest” were presented for 700 milliseconds prior to the beginning of each trial to indicate to the subjects which task to perform.
During the matching task, three three-digit numbers were presented serially followed by a 12-second delay and then a three-digit target stimulus. Subjects judged whether the target stimulus was one of the three numbers just presented in that trial by pressing a button box that recorded their responses. Subjects pressed one button with the index finger if they judged the target to be one of the three stimuli, and pressed another button with the middle finger if they believed the target did not match any of the three stimuli. During the multiplication task, the stimuli were four single-digit or low-value double-digit numbers presented serially followed by a 12-second delay and then a three- or four-digit target stimulus. Subjects judged whether the target number was the product of the four stimuli just presented during that trial. One button was pressed with the index finger if the subjects believed that the target stimulus was the product of the four stimulus numbers. The other button was pressed with the middle finger if the target was judged not to be the product of the four stimulus numbers. In the control (rest) task, subjects were asked to view the serial presentation of four zeros, but were told not to remember numbers or to perform calculations. When the target of “0000” appeared after the 12-second delay, subjects pushed a button with the index finger to control for motor activation involved in the response process. Subjects were asked to perform the calculations to the best of their ability. Accuracy was measured for all tasks. Stimuli were viewed through fiber-optic goggles adjusted by the subject prior to entering the magnet. Stimuli presentation was controlled by an Apple Macintosh Computer (Mountain View, CA) and the PsyScope experimental design package (Carnegie Mellon University, Pittsburg, PA).
Imaging
Functional MR imaging was performed on a 1.5-T imager (General Electric, Milwaukee, WI) equipped with resonant gradients (Advanced NMR, Wilmington, MA). Subjects lay supinely in the magnet with their heads immobilized by a neck support, foam wedges, and a restraining band drawn around the forehead. Scout images in the sagittal plane were acquired with parameters of 500/11 (TR/TE), a field of view of 24 centimeters, an imaging matrix of 256 × 192, and 5-mm contiguous sections. Eight anatomic images were acquired in an axial-oblique plane parallel to the anteroposterior commissures, with parameters of 500/11, a field of view of 40 centimeters, an imaging matrix of 256 × 192 and 8-mm-thick sections with a 1-mm gap. One hundred twenty activation images were collected at each of the same eight locations by using a single-shot, echo-planar, gradient-echo sequence with parameters of 1500/45, a flip angle of 60°, a field of view of 40 × 20 centimeters, an imaging matrix of 128 × 64, and 8-mm-thick sections with a 1-mm gap. Activation images were acquired while the subjects performed the above-described matching, multiplication, and control trials.
Data analysis
Prior to statistical analysis, the images from each run were motion corrected for three translation directions and for the three possible rotations by using the statistical parameter map (SPM)-96 program (40). The corrected images were spatially filtered using a gaussian filter with a full-width half-maximum (FWHM) value of 6.5 millimeters. The images were interpolated in time to account for the differences in the slice acquisition time within a run and filtered in the time domain by using a gaussian filter with a FWHM value of 1.5 seconds. The images taken at the same time relative to task onset were combined and compared with control images by using t-statistics corrected for linear drift (41). This created a statistical parametric map (SPM) for matching and multiplication tasks for each time point, with a temporal resolution of one TR interval (1.5 seconds). The SPMs and the anatomic images from individual subjects were transformed by in-plane transformation and slice interpolation into a normalized three-dimensional grid defined by Talairach and Tournoux (42).
The SPMs were not used to compute probabilities of activation because the number of degrees of freedom for the t-test is changed by autocorrelation (the MR signal is correlated in time), and autocorrelation is difficult to estimate. Instead, the SPMs were used as a derived measure of task-related activity to generate composite activation maps. To estimate the statistical significance of composite maps, we used the intersubject variability of the SPMs. We added the SPMs from individual subjects to obtain an activation measure (mean t-value) for each voxel. If there were no effect of the task, the expected value of the mean t-value across subjects would be zero. A straightforward estimate of significance could not be made because the distribution of the activation measure was not known. A randomization procedure was therefore used to generate the distribution of the task-related activation measures in order to estimate P values (43, 44). In this procedure, the sign of the t-values was reversed in half of the subjects. The mean value of the activation measure was then recalculated. Randomization was performed 1000 times, generating a distribution of the mean activation measure. The observed measure (mean t-value), calculated without sign reversal, was assigned a P value based on its position in this distribution. The proportion of times that the observed measure was more extreme than a randomized value represents a P value. It is the proportion of times we would expect to obtain a mean activation as large or larger than the one obtained if the null hypothesis (no effect) were true. The P value for each voxel was overlaid upon the mean anatomic image for display. The threshold used was P = .005 (uncorrected).
The button box responses for each subject were averaged across the 24 trials of the matching task and multiplication task, respectively. Differences in accuracy between multiplication and matching tasks were tested for significance with a paired Student's t-test.
Results
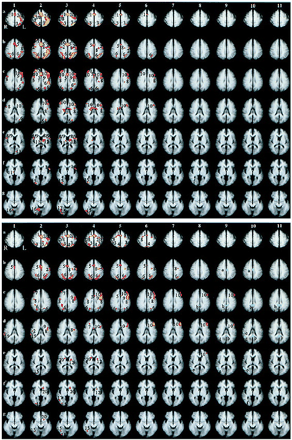
The matching task (Fig 1A) resulted in cortical activation in: the medial aspect of the superior frontal gyri; the anterior cingulate gyrus; the intraparietal sulcus; the right superior frontal sulcus; the middle frontal, inferior frontal, and precentral gyri (left greater than right); the anterior aspect of the left superior temporal gyrus; the thalamus; the basal ganglia (left greater than right); the lateral and inferior occipital gyri on the right; the left calcarine sulcus; and the posterior aspect of the right middle temporal gyrus. Activation in the left intraparietal sulcus, the left precental gyrus, and in the posterior aspect of the middle frontal gyrus on the left was seen approximately 6 seconds after the last stimulus was presented (Fig 1A, rows b–d, columns 3–6).
Regional brain activation during matching and multiplication tasks.
Top and Bottom, Composite activation maps comparing control trials with matching (Top) and multiplication trials (Bottom), respectively. Each column (1–11) represents composite images every 1.5 seconds. The first two columns were collected during stimulus presentation. Columns 3–10 represent the 12 seconds during which the subjects performed either the task or the control. The last column was collected during the subjects' response to target stimuli. Rows a–g indicate the slice position along the z -axis of the Talairach atlas system (50, 40, 32, 24, 12, 4, and−4, respectively). Numbers represent brain regions that were more active (P < .005, red-yellow color scale) during the task trials than during the control trials. Letters indicate brain regions that were more active (P< .005, blue-purple color scale) during the control trials than during the task trials. Both tasks produced a similar activation pattern in columns 2–6. The main differences in activation include a larger area of activation early during the matching task than during the multiplication task in the middle frontal gyrus bilaterally (A, rows c and d, columns 2–4), and during multiplication, activation in the posterior aspect of the left middle frontal gyrus (B, rows c and d, columns 3–9) was slightly delayed in onset and was seen for a longer period. Activation in the left intraparietal sulcus also lasted slightly longer during the multiplication task compared with the matching task (B, row b, columns 2–7). Areas of activation were: 1) superior frontal gyrus, 2) central sulcus region, 3) superior parietal lobule, 4) precuneus, 5) anterior cingulate gyrus and sulcus, 6) intraparietal sulcus (becomes superior occipital sulcus in row d), 7) superior frontal sulcus and adjacent gyri, 8) precentral sulcus and gyrus 9) inferior frontal gyrus and inferior frontal sulcus, 10) middle frontal gyrus 11) thalamus, 12) basal ganglia, 13) calcarine sulcus and cuneus, 14) anterior aspect of superior temporal gyrus, 15) lateral occipital gyrus, 16) lingual gyrus, 17) posterior aspect of middle temporal gyrus, 18) inferior occipital gyrus, 19) supramarginal gyrus, 20) head of caudate nucleus, (a) middle aspect of cingulate gyrus and sulcus, (b) posterior cingulate gyrus and sulcus, (c) postcentral gyrus (d) angular gyrus (e) posterior superior temporal sulcus and adjacent superior and middle temporal gyri and (f) lateral occipital gyri.
During the multiplication task, (Fig 1B), cortical activation was seen in brain regions similar to those active during the matching task, including: the medial aspect of the superior frontal gyri; the anterior cingulate gyrus; the left middle, inferior, and precentral gyri; the left basal ganglia; and the lateral occipital gyrus on the left. Compared with the matching task, the multiplication task resulted in additional activation in the head of the left caudate nucleus and early activation in the right supramarginal gyrus, smaller areas of early activation in the frontal lobes, especially in the right middle frontal gyrus and right superior frontal sulcus (Fig 1A and B, rows c and d, columns 2–4), and no early activation in the right precentral and inferior frontal gyri. There was also no activation in the thalamus, the anterior aspect of the left superior temporal gyrus, the left calcarine sulcus, and the right middle temporal gyrus (although there was activation in the adjacent lateral occipital gyrus). After the last stimulus was presented, activation in the left intraparietal sulcus was seen for approximately 7 seconds, and activation in the posterior aspect of the middle frontal gyrus on the left was visible for approximately 10 seconds (Fig 1B, rows c and d, columns 3–9).
Mean percentage accuracy across eighteen subjects (Table) in the matching and multiplication trials was 89 and 67, respectively (P < .005).
Occupation, math education, and average percent accuracy* on the fMR imaging tasks for eighteen subjects
Discussion
Composite time-course maps of brain activation show brain areas representing the neural networks involved with memory and multiplication of numbers. Multiplication engages a number of brain regions similar to those active during a memory task, including: the medial superior frontal gyri near the brain convexity; the anterior cingulate gyrus; the central sulcus region and precentral gyri on the left; the middle and inferior frontal gyri more on the left than the right; the intraparietal sulci bilaterally; gyri in the right occipital lobe; and the basal ganglia. These areas subserve neural processes relevant to visual recognition and transmission of number identity to other brain regions, storage and retrieval of numbers, number comparison, and semantic representation of numbers (1, 24, 25).
There were a few brain regions that activated in one task and not the other, but the main differences in activation patterns between the two tasks were the early activation in the frontal lobes, with more right frontal lobe involvement during the matching task than during the multiplication task and the longer activation period in the left frontal lobe during multiplication. The early activation of the right frontal lobe during the matching task included regions in the superior, middle, and inferior frontal gyri that are involved in a variety of language, memory, and attention tasks (45–51). Retrieval of verbal memory, which is a process known to involve the right hemisphere preferentially over the left, might account for part of the activation seen in the right frontal lobe (52).
Of the two tasks, only the multiplication task required subjects to calculate. Electrophysiologic studies (ie, ERP measures) show that parietal lobe neurons involved in calculation fire for a longer time (up to 800 milliseconds) compared with neurons involved in reading numbers (300–500 milliseconds) (39). The fMR time-course map of multiplication, which demonstrates persistent activation in the left middle frontal gyrus and slightly longer activation in the intraparietal sulci, may indicate activity across time of brain areas important to calculation in a way similar to, but much less exact than, the ERPs. The frontal lobe region is also active in language, attention, and working-memory tasks (45–51, 53). Our results suggest that memory and language-based strategies using verbal representation of numbers may be important components of mental arithmetic.
The activation differences between matching and multiplication tasks seen in the left middle frontal gyrus could also result from differences in task difficulty, as there was a significant difference in mean accuracy between the matching (89%) and the multiplication tasks (67%). Verbal rehearsal and memory of multiplication facts (the multiplication task) might represent a task that differs only quantitatively from verbal rehearsal and memory of numbers (the matching task). Additional studies with tasks more evenly matched on behavior scores will help determine the relative contributions of brain regions differentially activated during number processing.
Because the multiplication task in our study was difficult (only three subjects had an average multiplication score similar to the average matching score), it might have induced subjects to approximate the correct answer, so that the left frontal lobe sites might indicate activation from cognitive processes used in approximation. A study by Dehaene et al suggested that the language-based component of exact mental arithmetic resides in the inferior aspect of the left frontal lobe (25). That study required subjects to perform two tasks when viewing simple addition problems: calculate an exact answer or approximate an answer. During the exact-answer task, activation occurred in inferior aspects of the middle and inferior frontal gyri. The approximate task resulted in activation in the intraparietal sulci and in regions similar to our frontal lobe activation sites, including the left middle frontal gyrus and more superior aspects of the left inferior frontal gyrus.
Converging evidence from lesion, imaging, and ERP studies points to a number of brain regions that form the neural circuitry underlying cognitive processes used in mental calculation. Occipital lobe areas, including the fusiform and lingual gyri, play a role in both identifying alphabetical or digital strings and in transmitting them to areas involved in language production and retrieval of arithmetic facts (27). The parietal lobe is important in number comparison and calculation, especially when approximating answers, and is also activated during attention and working-memory tasks (24, 25, 45, 54). In the frontal lobe, there are sites crucial to encoding stimuli and retrieval of memorized facts, to executive function relevant to attention and decision making, and to the verbal representation of numbers necessary to perform calculations (45, 46, 48–54). The basal ganglia are involved in loops between the cortex, striatum, globus pallidus, and thalamus that might be related to motor control and memory (55, 56).
The involvement of language sites in mental calculation might provide a link to understanding the development of mathematical and reading skills in children. When learning arithmetic, children probably use strategies necessary for learning to read (57). On a cognitive level, the representation and retrieval of arithmetic facts in memory appear similar to the representation and retrieval of verbal information in semantic memory. Future studies might explore the possibility that reading- and mathematically disabled children share a common deficit in representation and retrieval of semantic information.
Conclusion
Multiplication and memory of numbers share an integrated network of brain regions, including the right occipital lobe, the left basal ganglia, the intraparietal sulci, the anterior cingulate gyrus, and several sites in the frontal lobe. The left frontal lobe, which is more active in a multiplication task than in a memory task, appears to have an important role in mental multiplication of visually presented numbers.
Footnotes
2 Address reprint requests to Robert K. Fulbright, MD, Section of Neuroradiology, Department of Radiology, Yale University School of Medicine, PO Box 208042, New Haven, CT 06520-8042.
References
- Received August 12, 1999.
- Accepted after revision January 12, 2000.
- Copyright © American Society of Neuroradiology