Abstract
BACKGROUND AND PURPOSE: This study assessed the in vivo delivery, retrievability, short-term patency, and cellular response to a new flexible endovascular stent system in a rabbit model. The stent is designed for delivery through a microcatheter and is fully retrievable with electrolytic detachment from a delivery wire.
METHODS: We successfully deployed nine stents (range of sizes, 2.5–4 mm diameter, 15–35 mm length) in six straight (carotid) and three angled (subclavian) arteries of six Chinchilla Bastard rabbits. Serial imaging was performed by using intravenous digital subtraction angiography (IVDSA), contrast-enhanced MR angiography (CEMRA), time-of-flight MR angiography (TOF), and CT-angiography 3 days and 4 weeks after stent deployment. Subjects were euthenized after 4 weeks (n = 5), and stents were removed for histologic analysis.
RESULTS: Stent deployment was feasible in all cases. After initial deployment, all stents could be fully retrieved within the microcatheter. The detachment zone and the distal stent marker were easily visible under fluoroscopy, and final detachment occurred reliably in all cases. We observed no procedural complications. Noninvasive imaging by using IVDSA, MR angiography, and CT angiography was feasible in this stent system and demonstrated all arteries patent and not narrowed at 3 days and 4 weeks, findings that were confirmed by histologic analysis.
CONCLUSION: This electrolytically detachable stent is promising as a treatment for intracranial arteries, because it can be delivered through microcatheters small enough for intracranial navigation. It is fully retrievable, thus providing greater control than currently available stents. Noninvasive imaging by using IVDSA, MR angiography, and CT angiography is feasible in this stent system and may be useful for follow-up. Further long-term data are needed.
Despite rapid advancement in the development of endovascular devices such as flexible microcatheters, new coil configurations, and embolic materials, wide-necked aneurysms remain a therapeutic challenge (1–6). Wide-necked aneurysms are difficult to treat solely with electrolytically detachable coils because of coil-loop herniation through the broad neck in the parent artery or reduced possibility of obtaining satisfactory coil packing and aneurysm thrombosis. Endovascular strategies for managing wide-necked aneurysms include the use of complex-geometry, three-dimensional coils, or balloon protection during coil embolization (1, 5–10). Another solution for the treatment of wide-necked aneurysms is the combined intravascular stent implantation to narrow the neck and prevent coil migration and subsequent coil placement through the interstices of the stent into the aneurysm. This technique was first validated by canine- and swine-model experimental studies (11–15). Since then, numerous case reports and case series have described the successful use of this technique (2, 4, 9, 10, 16–18).
A limitation for intracranial use of this technique, however, is that most currently available stents, usually coronary stents, have a large profile and are too stiff to negotiate tortuous vascular segments at the skull base and small-diameter intracranial vasculature (19–26). In addition, current stent technology is mainly balloon expandable and risks damaging a dysplastic segment of the artery.
With the development of micromachinery allowing refinement of existing devices, smaller and more flexible generations of stents are engineered. Recently, initial results of the clinical use of a new self-expandable flexible stent system especially designed for intracranial aneurysm treatment have been reported (10, 19).
The ideal stent for intracranial use would be highly flexible, atraumatic, and easily and accurately delivered and could be seen easily on fluoroscopy. Ideally, the stent should be fully retrievable, thus allowing repositioning, and, once implanted, the device should be stable in position, to maintain patency of the host vessel.
The purpose of our study was to assess in vivo the mode of delivery, retrievability, short-term patency, and cellular response to a new flexible endovascular stent system in a rabbit model. The stent is designed for delivery through a microcatheter small enough for intracranial catheterization. Stent placement is performed by detachment from a delivery wire effected by electrolysis, allowing complete retrieval and repositioning even after full deployment.
Methods
Stent System: Technical Specifications
The stent (Dendron-MTI, Bochum, Germany) is laser-cut from nitinol into a honeycomb pattern, folded, and then fused to a stainless steel delivery system, from which it is detachable, allowing complete retrieval and repositioning even after full deployment (Fig 1A, -B). It rolls like a cigarette, basically open, and overlaps in the axial direction if oversized or remains open if undersized. Stent detachment is accomplished by application of direct current (2 mA, 4–6 V, 30–60 seconds) by using the EDC-II coil-delivery system (Dendron-MTI). The stent can be manufactured in any diameter from 2.5 to 6.0 mm and any length from 10 to 35 mm. The wall thickness is 50–70 μ, creating minimal intrusion on the lumen of the target vessel. The outside diameter of the stent is small enough to allow delivery through a 0.021–0.027-inch inner-diameter microcatheter that can be used with any appropriate microguidewire. The stent in its original design has a radiopaque proximal marker (at the detachment zone). In the meantime, a second distal radiopaque marker has been added. Benchmark testing showed that the detachment zone practically could not be stretched or torn.
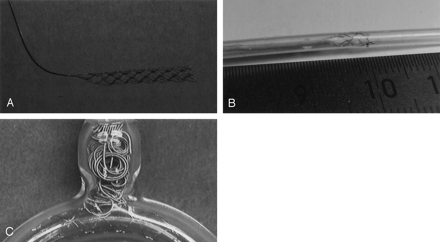
Illustrative benchmark testing of the stent system.
A, Prototype stent manufactured with 4-mm diameter. The platinum/nitinol alloy stent is attached to a steel delivery wire and detached by electrolysis of an uninsulated junction zone.
B, Stent partially retrieved into the microcatheter.
C, Deployed stent in an aneurysm glass model around a 90° bend mimicking basically a wide-necked carotid-ophthalmic aneurysm treated with coils.
Animal Preparation
Nine stents were deployed in the common carotid and subclavian arteries of six Chinchilla Bastard rabbits (3–5 kg body weight [bw]). All animal experimentation and handling were conducted in accordance with the national laws for animal protection and have been approved by the local review board for care of animal subjects.
Experimentation was performed under general anesthesia, induced by subcutaneous injection of ketamine (80 mg/kg bw) and xylazine (7 mg/kg bw), and maintained by intravenous administration of propofol (40 mg/kg bw/h). Animals were intubated and ventilated manually. By using a sterile technique, the right common femoral artery was surgically exposed, and a 5F vascular sheath was introduced in a retrograde manner. A 5F polyethylene catheter was advanced into the inominate artery. A 2F braided microcatheter and a Transend EX 14-guidewire was coaxially introduced into the target artery.
All angiographic procedures were performed on a Toshiba Infinix system. Before stent placement, diagnostic angiography of the target artery was performed. Stents were delivered via the microcatheter and placed in the right common carotid artery in each animal and additionally in the subclavian arteries of three animals. The roadmap technique was used for precise positioning of the stents. Both guide catheter and microcatheter were continuously flushed with sterile 0.9% saline to which 500 IU/L heparin had been added. Stents were placed, by first advancing the stent in its final position and then pulling back (unsheathing) the microcatheter while holding the stent in its position. To analyze stent retrievability after stent deployment, full retrieval of each stent within the microcatheter was attempted, after which the stent was placed again in the target vessel. Once in final position, the stent was detached from the delivery wire by electrolysis at the steel-platinum junction after application of a 2-mA direct current. Detachment time was recorded for each stent. Intraarterial control angiography was performed immediately after stent placement with two frames per second digital subtraction angiography (DSA) imaging in different planes.
Animals received 300 IU of heparin intravenously before stent placement. In addition, aspirin (50 mg/day) was started 2 days before stent implantation by intramuscular injection and was continued orally for 4 weeks.
The stents were sized to the estimated diameter of the target vessel, measured from the intraarterial angiogram after correction for radiographic magnification, by using a ruler overlying the vessel for reference. The vessels treated ranged from 2.5 mm to 4 mm in diameter. Stent sizes used ranged from 2.5 to 4 mm in diameter and 15 to 35 mm in length.
The three stents used in the first two animals had higher radial forces that have been decreased in the following devices. Stents used in animal 6 had two radiopaque markers, proximal at the detachment zone and an additional distal marker.
Imaging Protocol
Follow-up imaging was performed serially by using intravenous DSA (IVDSA), contrast-enhanced MR angiography (CEMRA), time-of-flight MR angiography (TOF), and CT-angiography 3 days and 4 weeks after stent deployment. For imaging, animals were anesthetized by subcutaneous injection of ketamine (80 mg/kg bw) and xylazine (7 mg/kg bw). All imaging was performed in one imaging session per time point.
Intravenous DSA
A 22G angiocatheter was placed in the left or right ear vein. Two frames per second DSA imaging was performed while injecting 4 mL of nonionic iodinated contrast material by hand into the ear vein.
MR Angiography
MR angiography was performed on a 1.5T MR scanner (amplitude, 40 mT/m; slew rate, 150 mT/m per ms) by using the standard head coil. Contrast-enhanced MR angiography was performed in a coronal angulation. Four flash sequences were started immediately after bolus injection of 0.2 mmol/kg bw gadolinium-diethylenetriamine pentaacetic acid via an ear vein. Scan parameters were TR/TE, 2.86/1.01 ms; flip angle, 25°; FOV, 175 × 280 mm; section thickness, 0.75 mm; sections per slab, 56; matrix, 512 × 160. TOF was obtained with magnetization transfer, flow compensation in all three axes, and variable bandwidths: TR/TE, 40/7.15 ms; flip angle, 25°; section thickness, 1 mm; FOV, 170 × 56.3 mm; sections per slab, 56; matrix, 512 × 144; 6.42 minutes acquisition time. For TOF, axial source images were acquired.
Postprocessing was performed on a Leonardo workstation. All MR angiograms were reconstructed in a three-dimensional mode with maximum intensity projections (MIPs) targeted on the vessels of interest with lateral rotation obtained by 10° increments. In addition, we performed multiplanar reconstructions (MPRs) to increase visualization of the stent and the parent artery.
CT Angiography
CT angiography was performed on a multisection helical CT scanner in all animals. We used a 1-mm collimation, pitch 1, the reconstructed section thickness was 1 mm. Bolus watching was used at the level of the heart. Ten milliliters of nonionic contrast material were infused via an ear vein by a power injector, with a flow of 1.5 mL/s. Scanning was started when maximal contrast enhancement was visible at bolus watching (usually after 4 seconds). Postprocessing was performed on a Leonardo workstation by using MIPs and coronal MPRs. MIPs and MPRs were included for data analysis.
Histologic Analysis
Subjects were euthenized after 4 weeks, and the stents, including 10–15 mm of the adjacent artery, were removed for macroscopic and histologic examination. After fixation with formaldehyde, the stents were separated from the vessel wall and prepared for microscopic examination.
Results
Stent Deployment: Feasibility
Difficulty was experienced in neither transferring the restrained device from the loading sheath to the microcatheter nor extending the device from the microcatheter. Stent deployment was technically feasible in all cases. For delivery, the stent was inserted into the microcatheter and advanced to the distal tip of the microcatheter. The stent was then unsheathed by retracting the microcatheter while holding the stent in place. By using this technique, accurate stent placement was reliably feasible in all cases.
Visibility
Radiopacity of the proximal (detachment zone) stent marker was good for all stents (Fig 2). Visibility of the stent itself, however, was poor, even by using high-quality fluoroscopy. Accordingly, a second distal radiopaque marker has been added in the recently manufactured stents (n=6). These two markers allowed easily visualization of the distal and proximal ends of the stent in addition to supporting accurate stent placement.
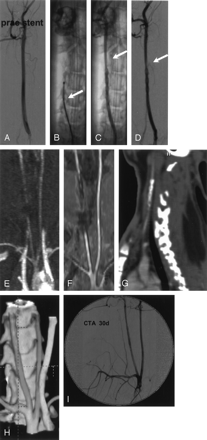
Multimodal imaging before and after stent placement.
A, Intraarterial angiogram of the right common carotid artery before stent placement.
B, Plain radiograph showing the stent partially deployed within the vessel. Note the junction zone still within the microcatheter (arrow).
C and D, Intraarterial angiogram (plain and unsubtracted) immediately after stent deployment. Note the slightly irregular vessel surface at the stent site and the slight distal narrowing (arrows) due to vasospasm.
E and F, MR angiography, TOF (E) and contrast-enhanced (F) 3 days after stent implantation. The nitinol stent induces artifacts mainly at the proximal stent site due to the marker.
G and H, CT angiography, sagittal reformation (G) depicts the stent. 3D images (H) demonstrating the open vessel. Note the slightly irregular vessel surface at the stent site.
I, Intravenous angiogram 4 weeks after stent deployment. Note the second stent placed into the right subclavian artery (diameter 2.5 mm, length 20 mm). Both vessels are open without narrowing.
Retrievability
To analyze stent retrievability, fully retrieval within the microcatheter of each stent was attempted after initial deployment. This was possible in all cases. Subsequent second stent deployment was feasible in all stents without problems. In our first three cases of stent deployment, we even retrieved each stent twice before final detachment. This was possible without any problem for all three stents.
Detachment
Detachment time was recorded for each stent and varied from 25 to 86 seconds. Detachment occurred reliably in all stents. Control angiography demonstrated all stented arteries to be patent with the stent fully opened without injury to the vessel wall.
Procedural Complications
We observed no procedural complications with stent implantation, especially no perforation or dissection of the vessels. No distal migration of the device was observed.
In the first animal, we observed a slightly irregular vessel surface and slight distal vessel narrowing (vasospasm) after stent placement, probably as a result of the high radial forces (Fig 2). With the modified stent system with reduced radial forces (animals 2–6), no such phenomenon and no vasospasms were observed.
Follow-Up Imaging
At control 3 days and 4 weeks later, all arteries remained widely patent and unnarrowed; patency was not related to vessel or stent size. No migration or kinking of the stents was noted.
Serial noninvasive imaging by using intravenous DSA, MR angiography, and CT angiography proved feasible in this stent system. In TOF, as well as contrast-enhanced MR angiography, the nitinol stents caused signal intensity void, mainly at the proximal and distal stent site (markers). Nevertheless, patency of the artery could be inferred from the observation of signal intensity proximal and distal to the stent. CT angiography did not reveal artifacts due to the stent and demonstrated patency of all stented arteries.
Histologic Analysis
For microscopic examination, the stents were removed from the arterial segment, leaving an easily visible impressions of the former stent site in the arterial wall. We found the target vessels patent in all cases. Detailed examination showed minimal organization of stent surface associated with partial (postmortem) thrombus in two cases. No perforation of the vessels occurred. The expansile character of the stent resulted in slight stretching of the elastic lamina and slight dilatation of the artery, which was more prominent in the first case.
Hematoxilin-eosin staining revealed a slight, very smooth intimal proliferation of endothelial-like cell formations at the luminal surface of the stent site, resulting in a probably flow-associated flattening of the arterial wall impressions. This represents a zonal chronical foreign-body reaction, resulting in a smooth internal vessel surface without narrowing of the vessel lumen.
Discussion
Stents have been used widely in occlusive lesions in the peripheral, renal, and coronary arteries to treat stenosis of vessels narrowed by a variety of pathologic conditions (27–31). Initially used mainly in extracranial cerebral vessels for carotid artery stenosis or the treatment of pseudoaneurysms of the extracranial carotid artery (2, 5, 21, 32–34), small stents are now increasingly used for intracranial vessel disease such as the treatment of wide-necked aneurysms not amenable to conventional endovascular techniques (10, 19, 25, 26). The stents are usually used in combination with detachable coils delivered through the stent struts to induce aneurysm thrombosis, with the additional benefit of the stent bridging the aneurysm neck to prevent coil migrating into the parent artery (5, 8–10). Major limitations of the currently available stents, usually cardiac stents, however, are their relatively stiffness, rendering them not flexible enough to pass the C1/C2 vertebral artery or carotid siphon tortuousities.
The design constraints for the device used in this study were to develop an endovascular stent that is flexible enough to be delivered via a microcatheter and to be placed in small vessels but with sufficient radial forces to conform to the vessel wall when deployed. In addition, the stent should be fully retrievable, and, once implanted, the device should be stable in position, to maintain patency of the host vessel.
The purpose of our experimental study was to evaluate in vivo the mode of delivery, retrievability, short-term patency, and cellular response to a new flexible endovascular neurostent system in a rabbit model. The animal model and the two arterial sites used for stent placement were chosen because of their relative similarity in size to human cerebral arteries (35). Cerebral arteries in other commonly used experimental animals are too small. In addition, the rabbit represents the species with the greatest homology to the hematologic and coagulation system of humans (36).
Successful and accurate stent deployment was technically feasible in all cases. Preliminary in vitro tests established the optimum delivery catheter. By using other microcatheters, delivery was difficult because of increased friction between the stent and the wall of the catheter. Friction between the catheter and stent may become a problem when vessel anatomy is very tortuous, an effect only partially simulated in this experiment.
Deployment of the stent is accomplished by unsheathing the stent by pulling back the microcatheter while holding the stent in place. Thus, in contrast to coils, which are pushed out of the catheter, no mechanical force is necessary to deliver the stent. The radial force of the stent is much more less than that of balloon-expandable stents used in cardiology and comparable to a commercially available stent system (Neuroform; Boston Scientific, Natick, MA) especially designed for the treatment of wide-necked cerebral aneurysms.
In our stent system, no core wire is necessary. This is in contrast to all other stents used for cerebrovascular disease, which are deployed by an over-the-wire technique. Leaving the guidewire in place after stent deployment in curved vessels might be an option to stabilize the stent and thus prevent stent displacement while catheterizing the aneurysm with a second microcatheter. In contrast to the Neuroform, because of the design of our stent displacement should not be an issue. Clinical data, however, are necessary to answer this issue. We think the lack of a core wire is a clear advantage, making the system very easy for a single interventionalist to handle. Another advantage is that the aneurysm can be catheterized with the microcatheter immediately after stent deployment.
Stent placement is performed by electrolytic detachment, allowing complete retrieval and repositioning even after full deployment. Retrievability of the stent system, which was possible in all stents is a unique feature making stent deployment more controllable and its use potentially safer. In an experimental study, Byrne et al evaluated an electrolytically detachable coil system configured as an endovascular stent, which might be used for intracranial vessels (19). No further reports on that appealing technology, however, are currently available.
Theoretically, retrievability of the stent might also allow remodeling of wide-necked aneurysms without temporary vessel occlusion such as with the balloon remodeling technique (37). After coil packing of the aneurysm, the stent might then be removed by first pushing the microcatheter over the stent and then retrieving the stent within the microcatheter. In the clinical setting, however, there is a conceivable risk to retrieve coils while pushing back the microcatheter over the stent if they adhere to the stent struts.
Detachment occurred reliably in all stents. Radiopacity of the proximal (detachment zone) stent marker was good for all stents. Visibility of the stent itself, however, was poor even when high-quality fluoroscopy was used. These limitations of fluoroscopy in visualization of the stent are well known. Especially when used in combination with coils, distinguishing coil protrusion into the parent artery from aneurysmal coil mass superimposing the stent may be problematic. Accordingly, a second distal radiopaque marker has been added in the recently manufactured stents. These two markers allowed easily visualization of the distal and proximal ends of the stent, thus enabling accurate stent placement.
At follow-up at 3 days and 4 weeks, all arteries were patent and not narrowed. Follow-up imaging of patients undergoing endovascular therapy for an intracranial aneurysm is crucial. Minimally invasive imaging techniques such as MR angiography and CT angiography are especially of interest. Previous reports have documented the capabilities and limitations of depicting aneurysms before and after treatment with aneurysm clips and coils (38–40). Vascular MR imaging, however, is expected to be compromised by the presence of metallic endovascular implants such as stents.
In our study, all stents caused signal intensity void mainly at the proximal and distal stent site either by using TOF as well as contrast-enhanced MR angiography. Nevertheless, patency of the artery could be inferred from the observation of signal intensity proximal and distal to the stent. In contrast, stents did not cause major artifacts on CT angiography and patency of the artery was visible in all cases, which suggests that this imaging technique might be useful for follow-up imaging. This issue, however, warrants further investigation.
We observed no procedural complications. Because most procedure-related complications are of thromboembolic nature, intraprocedural anticoagulation therapy is of great importance in this setting. We used intraprocedural intravenous heparin in all animals, and aspirin was started 2 days before stent implantation and continued daily throughout the study protocol. In a preliminary in vivo experiment, we observed severe stent and vessel thrombosis with the death of the animal 1 day after stent placement due to an insufficient anticoagulation management.
Probably as a result of the high radial forces, we observed slightly irregular vessel surfaces and a slight distal vasospasm after stent placement on control angiogram, a finding also reported by other experimental groups for this animal model (41). With modification of the stent system with reduced radial forces, however, no such phenomenon and no vasospasms were observed.
A factor to be considered after endovascular stent placement is the risk of delayed restenosis due to intimal hyperplasia. We did observe only a very slight, smooth layer of intimal hyperplasia. Because there was no narrowing of the vessel lumen in all cases, quantification of intimal hyperplasia by using intimal–media ratios or cross-sectional analysis was not performed in this preliminary study; however, the relatively short observation period might be a limitation. Further angiographic and histologic long-term follow-up is necessary to define this issue.
Another limitation of our study is that we used healthy animals without pathologic vessel alterations such as arterosclerosis. Damage is more likely to occur in diseased vessels such as those narrowed by vasospasm or atherosclerosis. How this novel stent system performs in pathologic vessels remains to be seen.
Technically, this retrievable stent can be manufactured in different designs such as varying length, diameter, or radial forces. A tapered design with smaller sizes might be also applicable for smaller vessels such as segments M1, M2 or A1, A2, where occlusion of distal branches cannot be tolerated. Because the stent is cut from nitinol sheet, it is possible to vary the strut sizes and even to have a nonuniform distribution of fenestrations. Theoretically, this might offer additional possibilities in aneurysm treatment such as bare placement of a stent with struts sufficiently attenuated to reduce flow within wide-necked aneurysms and thus promote aneurysm thrombosis.
Such issues, however, must be addressed in further studies such as in the elastase-induced aneurysm model in rabbits, an animal model, closely representing morphology and hemodynamics of human cerebral aneurysms and shown to be suitable for testing new endovascular occlusion devices (42–44).
Additional indications such as stenosis or dissection might theoretically be amenable to this stent system. Preliminary testing of postdeployment angioplasty has shown promise.
Conclusion
Our preliminary results demonstrate that this new device shows promise as a stent for intracranial arteries because it can be accurately and reliably positioned in small vessels with good midterm patency during follow-up. In addition, this electrolytically detachable stent is fully retrievable and thus provides greater control than do currently available self-or balloon-expandable stents. Noninvasive imaging by using MR angiography and CT angiography is feasible in this stent system and may be useful for follow-up monitoring. Nevertheless, further studies especially focusing on long-term data including histologic analysis are necessary.
Acknowledgments
This study contains parts of W. Becker’s doctoral thesis. The study was supported by a grant from the Foundation Program of Nordrhein-Westfalen, Germany and the European Union. Dr. Doerfler is a proctoring consultant to Dendron/MTI. We thank our technicians of the angiogaphy team, especially A. Fischer-Schramm and S. Lindner, for their excellent support.
References
- Received April 27, 2004.
- Accepted after revision August 24, 2004.
- American Society of Neuroradiology