Abstract
BACKGROUND AND PURPOSE: No qualitative imaging feature currently predicts molecular alterations of pediatric low-grade gliomas with high sensitivity or specificity. The T2-FLAIR mismatch sign predicts IDH-mutated 1p19q noncodeleted adult gliomas with high specificity. We aimed to assess the significance of the T2-FLAIR mismatch sign in pediatric low-grade gliomas.
MATERIALS AND METHODS: Pretreatment MR images acquired between January 2001 and August 2018 in pediatric patients with pediatric low-grade gliomas were retrospectively identified. Inclusion criteria were the following: 1) 0–18 years of age, 2) availability of molecular information in histopathologically confirmed cases, and 3) availability of preoperative brain MR imaging with non-motion-degraded T2-weighted and FLAIR sequences. Spinal cord tumors were excluded.
RESULTS: Three hundred forty-nine patients were included (187 boys; mean age, 8.7 [SD, 4.8] years; range, 0.5–17.7 years). KIAA1549–B-Raf proto-oncogene (BRAF) fusion and BRAF p.V600E mutation were the most common molecular markers (n = 148, 42%, and n = 73, 20.7%, respectively). The T2-FLAIR mismatch sign was present in 25 patients (7.2%). Of these, 9 were dysembryoplastic neuroepithelial tumors; 8, low-grade astrocytomas; 5, diffuse astrocytomas; 1, a pilocytic astrocytoma; 1, a glioneuronal tumor; and 1, an angiocentric glioma. None of the 25 T2-FLAIR mismatch pediatric low-grade gliomas were BRAF p.V600E–mutated. Fourteen of 25 pediatric low-grade gliomas with the T2-FLAIR mismatch sign had rare molecular alterations, while the molecular subtype was unknown for 11 tumors.
CONCLUSIONS: The T2-FLAIR mismatch sign was not observed in the common molecular alterations, BRAF p.V600E–mutated and KIAA1549-BRAF fused pediatric low-grade gliomas, while it was encountered in pediatric low-grade gliomas with rare pediatric molecular alterations.
ABBREVIATIONS:
- DNET
- dysembryoplastic neuroepithelial tumor
- pLGG
- pediatric low-grade glioma
- RAS/MAPK
- RAS-mitogen-activated protein kinase
- TKDD
- tyrosine kinase domain duplication
Pediatric low-grade gliomas (pLGGs) are the most common pediatric brain tumors, accounting for approximately 40% of all pediatric brain tumors.1,2 They are a heterogeneous group of tumors classified by the World Health Organization as grade 1 or 2.3 Molecular profiling studies have identified genetic events in pLGGs involving the RAS-mitogen-activated protein kinase (RAS/MAPK) pathway.4 Most commonly, these are events involving B-Raf proto-oncogene (BRAF) or germline neurofibromatosis 1 (NF1) alterations.5,6 Rarer alterations affect RAS/MAPK signaling, including fibroblast growth factor receptor (FGFR)1/2/3, ROS1, and non-RAS/MAPK alterations, such as MYB, MYBL1, IDH1, and others.1,4 To date, no qualitative imaging features exist predicting any of the pLGG molecular alterations with high sensitivity or specificity.
In 2017, Patel et al7 described the T2-FLAIR mismatch sign in a sample of 125 adult lower-grade gliomas to predict IDH-mutated 1p19q noncodeleted gliomas with high specificity. It is defined by hyperintense signal on T2-weighted sequences and hypointense signal on FLAIR sequences with a hyperintense peripheral rim. This imaging feature has since gained increasing attention in adult neuroradiology due to the wide availability of T2-weighted and FLAIR sequences. Currently, there is only anecdotal evidence of the T2-FLAIR mismatch sign in the pediatric population. In 2019, Johnson et al8 reported 5 false-positive instances of T2-FLAIR mismatch, 4 of which occurred in children and 3 of which were neoplastic. These included a pilomyxoid astrocytoma, a H3K27M-mutant midline glioma, and a low-grade astrocytoma with an MYB rearrangement.8 To our knowledge, no prior study has systematically investigated the occurrence of the T2-FLAIR mismatch sign in pLGG.
We therefore aimed to assess the occurrence of the T2-FLAIR mismatch sign in pLGG in a large pediatric institutional cohort.
MATERIALS AND METHODS
Patient Sample
This retrospective study was approved by the local institutional review board (The Hospital for Sick Children, Toronto). Because of the retrospective nature of the study, informed consent was waived by the local research ethics board. All patients were identified from the electronic health record database between January 2001 and August 2018. Patient inclusion criteria were the following: 1) 0–18 years of age, 2) the availability of molecular information in histopathologically confirmed pLGG as defined by Louis et al,9 and 3) the availability of preoperative brain MR imaging with non-motion-degraded T2-weighted and FLAIR sequences. Spinal cord tumors were not included in this study.
Molecular Analysis
BRAF fusion status was determined using an nCounter Metabolic Pathways Panel (NanoString Technologies) or fluorescence in situ hybridization, while the BRAF p.V600E mutation was determined using immunohistochemistry or droplet digital polymerase chain reaction. Additional alterations were detected by RNA panel sequencing as previously described.4 For most patients, molecular analysis was performed with formalin-fixed paraffin-embedded tissue that was obtained at the time of the tissue sampling.
MR Imaging
All patients underwent MR imaging of the brain at 1.5T or 3T across various vendors (Signa, GE Healthcare; Achieva, Philips Healthcare; Magnetom Skyra, Siemens). The standardized sequence protocol at our institution included the following sequences: a sagittal 3D T1-weighted sequence with axial and coronal reformats, an axial DWI, an axial and/or coronal FLAIR sequence, an axial and/or coronal and/or sagittal T2-weighted sequence, an axial SWI or multiplanar gradient recalled acquisition, a gadolinium-based contrast agent–enhanced axial 3D T1-weighted sequence with coronal and sagittal reformats, and a gadolinium-based contrast agent–enhanced axial or coronal T1-weighted spin-echo sequence. All MR imaging data were extracted from the PACS, de-identified for further analyses, and transferred to an off-line workstation.
Analysis of the Study Cohort and Statistical Analysis
Two pediatric neuroradiologists (M.W.W. with 4 years of experience after a pediatric neuroradiology fellowship and B.B.E.-W. with >15 years of postfellowship experience) reviewed baseline brain MR images of all included patients, blinded to clinical and molecular data. Signal characteristics of brain tumors were reviewed, and the presence of the T2-FLAIR mismatch sign was noted on the basis of the description by Jain et al.10 Additionally, images were reviewed for the presence of hemosiderin on SWI and multiplanar gradient recalled sequences. Decisions were reached in consensus.
Descriptive statistical analyses were performed using the Statistical Package for the Social Sciences software (SPSS), Version 18 (IBM). For baseline characteristics, continuous data were presented using mean (SD), and categoric data were presented using integers and percentages.
RESULTS
Patient Demographics, Histopathologic Diagnoses, and Molecular Markers
A total of 389 MR images of 389 patients were available for analysis. Of these, 40 MR images were excluded due to a nonavailable FLAIR sequence (n = 28), posttherapeutic status (n = 5), tumor extending into the cervical cord (n = 3), nonavailable molecular information (n = 2), a nonavailable T2-weighted sequence (n = 1), and motion degradation of the FLAIR sequence (n = 1). The inclusion criteria were fulfilled by 349 patients (187 boys, 53.6%). The mean age at the time of diagnosis was 8.7 (SD, 4.8) years (range, 0.5–17.7 years).
The sample consisted of biopsy-proved pilocytic astrocytomas (n = 151), low-grade astrocytomas (n = 66), gangliogliomas (n = 43), dysembryoplastic neuroepithelial tumors (DNETs) (n = 24), diffuse astrocytomas (n = 22), pilomyxoid astrocytomas (n = 11), pleomorphic xanthoastrocytomas (n = 7), oligodendrogliomas (n = 7), glioneuronal tumors (n = 5), angiocentric gliomas (n = 5), desmoplastic infantile gliomas (n = 2), neurocytomas (n = 2), mixed tumor components (n = 2), gangliocytoma (n = 1), and polymorphous low-grade glioneuronal tumor of the young (n = 1). There were no IDH-mutant 1p/19q noncodeleted gliomas.
KIAA1549-BRAF fusion was the most common molecular marker (n = 148). Less common alterations were BRAF p.V600E mutation (n = 73), FGFR1 tyrosine kinase domain duplication (TKDD) (n = 7), NF1 (n = 11), FGFR1-TACC1 fusion (n = 6), alterations in FGFR2 (n = 6), MYB (n = 5), MYBL1 (n = 4), FGFR1-N546K (n = 1), mutation of IDH1 (n = 3), alteration of MET N375S (n = 2), QKI-RAF1 (n = 2), BRAF V600ins (n = 2), FGFR1 deletion (n = 1), FGFR4 (n = 1), BRAF D594N and KRAS Q61R (n = 1), BRAF-FAM131B (n = 1), CLCN6-BRAF (n = 1), FYCO-RAF1 (n = 1), fusion of GOPC-ROS1 (n = 1), alterations in KIT V825I (n = 1), KRAS-Q22K (n = 1), MAP2K1 (n = 1), MET H1112Y (n = 1), MID1-NTRK2 (n = 1), mutation of MYB-QKI (n = 1), fusion of PDGFB-LRP1 (n = 1), and alteration of PDGFRA-K385M (n = 1), PIK3CA Q60K (n = 1), RET D892N (n = 1), SF3B1-NTRK2 (n = 1), TAX1BP1-BRAF (n = 1), FGFR1-K654N, T656P (n = 1), and FGFR1-K646E (n = 1). A total of 56 children with pLGG had incomplete test panels. Of those, 50 were negative for KIAA1549-BRAF fusion, while no test result was available for 6 tumors. Fifty-three were negative for the BRAF p.V600E mutation, while no test result was available for 3 tumors. Last, 52 were negative for both FGFR1-N546K alteration and mutation of IDH1, while no test result was available for 4 tumors, respectively.
Analysis of T2-FLAIR Mismatch Sign
The T2-FLAIR mismatch sign was observed in 25 of 349 patients with pLGG (7.2%). Seventeen patients were boys (68%). The mean age was 8.3 (SD, 4.8) years (range, 1–16 years). The T2-FLAIR mismatch sign was encountered in the frontal lobe (n = 10), temporal lobe (n = 7), parietal lobe (n = 3), thalamus (n = 2), brainstem (n = 2), and intraventricularly (n = 1). Of the 25 pLGGs with the T2-FLAIR mismatch sign, 9 were DNETs (36%), 8 were low-grade astrocytomas (32%), 5 were diffuse astrocytomas (20%), 1 was a pilocytic astrocytoma (4%), 1 was a glioneuronal tumor (4%), and 1 was an angiocentric glioma (4%).
In relation to all patients reviewed, the T2-FLAIR mismatch sign was found in 9 of 24 DNETs (37.5%), 5 of 22 diffuse astrocytomas (22.7%), 1 of 5 glioneuronal tumors (20%), 1 of 5 angiocentric gliomas (20%), 8 of 66 low-grade astrocytomas (12.1%), and 1 of 151 pilocytic astrocytomas (0.7%).
In relation to all patients reviewed, the T2-FLAIR mismatch sign was found in 3 of 4 MYBL1 pLGGs (75%, Fig 1), 2 of 7 FGFR1-TKDD pLGGs (28.6%), 2 of 6 FGFR1-TACC1 pLGGs (33.3%, Fig 2), 1 of 5 MYB pLGGs (20%), 1 of 1 FGFR4 pLGGs (100%, Fig 3), 1 of 1 GOPC-ROS1 pLGGs (100%), 1 of 3 IDH1 pLGGs (33.3%), 1 of 2 MET N375S pLGGs (50%), 1 of 1 MYB-QKI pLGG (100%, Fig 4), and 1 of 1 PDGFB-LRP1 pLGG (100%). Among the 11 tumors with incomplete test panels, all were negative for the BRAF p.V600E mutation, FGFR1-N546K alteration, and a mutation of IDH1. Nine of 11 tumors were also negative for KIAA1549-BRAF fusion, while no test result was available for 2 tumors.
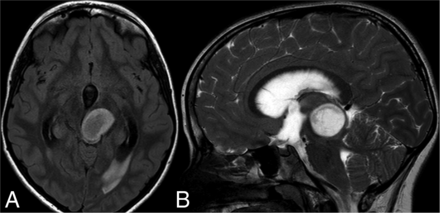
A 13-year-old boy with a left temporal diffuse astrocytoma with an MYBL1 alteration and T2-FLAIR mismatch sign. A, axial FLAIR and B, coronal T2.
A 10-year-old girl with a midline low-grade astrocytoma with FGFR1-TACC fusion and the T2-FLAIR mismatch sign. A, axial FLAIR and B, coronal T2.
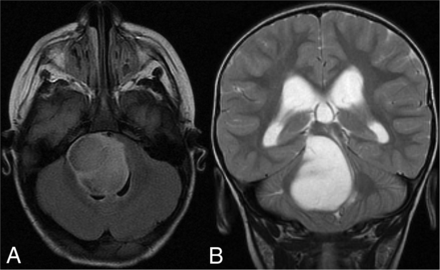
A 9-year-old boy with a left temporal DNET with an FGFR4 alteration and the T2-FLAIR mismatch sign. A, axial FLAIR and B, sagittal T2.
A 2-year-old boy with a brainstem low-grade astrocytoma with MYB-QKI fusion and the T2-FLAIR mismatch sign. A, axial FLAIR and B, axial T2.
Notably, no pLGG with the T2-FLAIR mismatch sign was BRAF p.V600E–mutated, and at least 23 of 25 tumors with a T2-FLAIR mismatch sign were also negative for KIAA1549-BRAF fusion (Online Supplemental Data). The Online Supplemental Data show the distribution of the histopathologic diagnoses of the pLGGs with a T2-FLAIR mismatch sign according to their molecular markers.
No abnormal susceptibility was found in any of the 25 pLGGs with the T2-FLAIR mismatch sign.
DISCUSSION
Using a large institutional cohort, we aimed to assess the occurrence of the T2-FLAIR mismatch sign in pLGGs and its relation to histopathologic and molecular markers. We found the T2-FLAIR mismatch sign to be absent in KIAA1549-BRAF–fused and BRAF p.V600E–mutated pLGGs. The sign was more commonly noted in rare molecular markers including MYBL1 pLGG, FGFR1-TKDD pLGG, FGFR1-TACC1 pLGG, MYB pLGG, FGFR4 pLGG, GOPC-ROS1 pLGG, IDH1 pLGG, MET N375S pLGG, MYB-QKI pLGG, and PDGFB-LRP1 pLGG. Fifty-six tumors had incomplete test panels. However, the most common molecular alterations (KIAA1549-BRAF fusion and BRAF p.V600E mutation) in addition to FGFR1-N546K alteration and mutation of IDH1 were excluded in almost all tumors. Of these 56 tumors, 11 (19.6%) demonstrated the T2-FLAIR mismatch sign.
In their recent meta-analysis, Do et al11 systematically reviewed the predictive accuracy of the T2-FLAIR mismatch sign in 1342 adults (age range, 19–82 years) with low-grade gliomas. The sign was found to have a pooled sensitivity of 40% and pooled specificity of 100% for IDH-mutant, 1p/19q noncodeleted low-grade gliomas. While the sign has been established in the adult neuroradiology literature, to our knowledge, its significance has never been assessed in a pediatric brain tumor cohort, likely due to greater diversity of histopathology, imaging morphology, and tumor location of pediatric gliomas compared with adult IDH-mutant, 1p/19q noncodeleted low-grade gliomas reported by Patel et al.7
The mismatch sign is less frequently encountered in the pediatric age group. However, there is anecdotal evidence of a false-positive T2-FLAIR mismatch sign in children. A prior report by Johnson et al8 mentioned a false-positive T2-FLAIR mismatch sign in the following: 1) a pilomyxoid astrocytoma in a 2-year-old child, 2) a non-neoplastic lesion (heterotopic gray matter) in a 12-year-old child, 3) a H3K27M-mutant midline glioma in a 14-year-old adolescent, and 4) a low-grade astrocytoma harboring MYB rearrangement in an 18-year-old individual. In addition, Kalelioglu et al12 reported the T2-FLAIR mismatch sign in 1 of 2 pediatric-type diffuse low-grade gliomas with a MYB/MYBL1 alteration. In our cohort of 352 pLGGs, we found 25 tumors with a T2-FLAIR mismatch (7.1%). This finding raises the question of the significance of the sign in the pediatric population. Unlike Johnson et al, we did not find the sign in pilomyxoid astrocytomas, but we observed the T2-FLAIR mismatch sign in 1 of 5 pLGGs with MYB and 3 of 4 pLGGs with a MYBL1 rearrangement. MYB and MYBL1 serve as transcriptional regulators important for cell proliferation and differentiation.1 They are hypothesized to reflect a single tumor entity13 and represent a distinct group within the pediatric-type diffuse low-grade gliomas in the 2021 WHO Classification of Tumors of the Central Nervous System.3 Furthermore, the T2-FLAIR mismatch sign was present in 2 of 7 FGFR1-TKDD pLGGs (28.6%) and 2 of 6 FGFR1-TACC1 fusion pLGGs (33.3%), which represented 2 diffuse astrocytomas and 2 low-grade astrocytomas histopathologically.
Other rare pLGG subtypes demonstrating the T2-FLAIR mismatch sign included the following molecular markers: FGFR4 alteration (DNET), GOPC-ROS1 fusion (pilocytic astrocytoma), MET N375S alteration (DNET), IDH1 mutation, MYB-QKI fusion, and PDGFB-LRP1 fusion (all low-grade astrocytomas). Regarding the commonly encountered histopathologic tumor types in pLGG, only 1 of 151 pilocytic astrocytomas (0.7%), 8 of 66 low-grade astrocytomas (12.1%), and none of 43 gangliogliomas demonstrated the T2-FLAIR mismatch sign. Instead, rarer histopathologies were enriched when encountering the T2-FLAIR mismatch sign, including 9 of 24 DNETs (37.5%), 5 of 22 diffuse astrocytomas (22.7%), 1 of 5 glioneuronal tumors (20%), and 1 of 5 angiocentric gliomas (20%). The rarity of the T2-FLAIR mismatch sign in common histologic tumor and molecular subtypes of pLGG suggests its potential as a diagnostic indicator for uncommon pLGG types.
Prior reports on IDH-mutant, 1p/19q noncodeleted low-grade gliomas in adults have demonstrated a strong association between the T2-FLAIR mismatch sign and intratumoral microcystic components.7,14⇓-16 This association can likely be attributed to the very long T1- and T2-relaxation times, which are the underlying MR imaging signal characteristics of the mismatched area.17 Of our 25 pLGGs with T2-FLAIR mismatch, 9 were DNETs, a tumor entity known to demonstrate a microcystic pattern histologically.18 It is difficult to say whether the microcystic pattern is related to the presence of the sign in other cases of our cohort, including low-grade, diffuse, and pilocytic astrocytomas and glioneuronal and angiocentric gliomas. The answer would require a histopathologic analysis of these cases beyond the scope of this study. While the underlying histopathologic characteristics of the T2-FLAIR mismatch sign in rare pLGG remain to be elucidated, we did not find the T2-FLAIR mismatch in the 2 most common types of molecular alterations of pLGG, KIAA1549-BRAF fusion and BRAF p.V600E mutation.
Several limitations need to be considered when interpreting the results of our study. First, 2 expert readers subjectively assessed the MR images in consensus, blinded to the molecular marker. Second, there were relatively few pLGGs with rare molecular markers in our sample. Consequently, the significance of the T2-FLAIR mismatch sign in GOPC-ROS1, IDH1, MET N375S, MYB-QKI, and PDGFB-LRP1 requires further clarification. Third, 56 tumors had incomplete test panels. Of these, 11 demonstrated the T2-FLAIR mismatch sign. While it would be interesting to know whether there are rare pLGG molecular subtypes present in this group, we were able to exclude the most common molecular alterations (KIAA1549-BRAF fusion and BRAF p.V600E mutation) in addition to the FGFR1-N546K alteration and the mutation of IDH1. Additional molecular analysis in the future may help elucidate the significance of the sign in rare subtypes of pLGG.
CONCLUSIONS
In a large institutional cohort of patients with pLGG, we assessed the occurrence of the T2-FLAIR mismatch sign and its relation to histopathologic and molecular markers. We found that the T2-FLAIR mismatch sign is absent in KIAA1549-BRAF–fused and BRAF p.V600E–mutated pLGG. The sign was encountered in rare molecular markers of pLGG, including MYB/MYBL1, MYB-QKI, FGFR1-TKDD, FGFR1-TACC1, FGFR4, IDH1, GOPC-ROS1, MET N375S, and PDGFB-LRP1. Due to the rarity of the T2-FLAIR mismatch sign in common histologic tumor types and molecular subtypes of pLGG, it could serve as a diagnostic marker for infrequent pLGG types.
Footnotes
C. Hawkins was supported by the Canadian Cancer Society (grant No. 702296) and the Canadian Institute of Health Research (grant No. 159805).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received December 16, 2022.
- Accepted after revision May 22, 2023.
- © 2023 by American Journal of Neuroradiology